Leukocyte and Dried Blood Spot Arylsulfatase A Assay by Tandem Mass Spectrometry
- PMID: 31922725
- PMCID: PMC7203001
- DOI: 10.1021/acs.analchem.9b05274
Leukocyte and Dried Blood Spot Arylsulfatase A Assay by Tandem Mass Spectrometry
Abstract
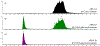
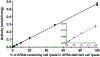
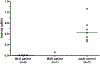
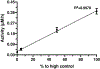
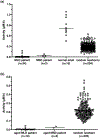
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays were developed to measure arylsulfatase A (ARSA) activity in leukocytes and dried blood spots (DBS) using deuterated natural sulfatide substrate. These new assays were highly specific and sensitive. Patients with metachromatic leukodystrophy (MLD) and multiple sulfatase deficiency (MSD) displayed a clear deficit in the enzymatic activity and could be completely distinguished from normal controls. The leukocyte assay reported here will be important for diagnosing MLD and MSD patients and for monitoring the efficacy of therapeutic treatments. ARSA activity was measured in DBS for the first time without an antibody. This new ARSA DBS assay can serve as a second-tier test following the sulfatide measurement in DBS for newborn screening of MLD. This leads to an elimination of most of the false positives identified by the sulfatide assay.
Figures

References
-
- Sessa M; Lorioli L; Fumagalli F; Acquati S; Redaelli D; Baldoli C; Canale S; Lopez ID; Morena F; Calabria A, Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. The Lancet 2016, 388 (10043), 476–487. - PubMed
-
- Biffi A; Montini E; Lorioli L; Cesani M; Fumagalli F; Plati T; Baldoli C; Martino S; Calabria A; Canale S, Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013, 341 (6148), 1233158. - PubMed
-
- Lorioli L; Cesani M; Regis S; Morena F; Grossi S; Fumagalli F; Acquati S; Redaelli D; Pini A; Sessa M; Martino S; Filocamo M; Biffi A, Critical issues for the proper diagnosis of Metachromatic Leukodystrophy. Gene 2014, 537 (2), 348–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

