PKCβ and reactive oxygen species mediate enhanced pulmonary vasoconstrictor reactivity following chronic hypoxia in neonatal rats
- PMID: 31922892
- PMCID: PMC7052628
- DOI: 10.1152/ajpheart.00629.2019
PKCβ and reactive oxygen species mediate enhanced pulmonary vasoconstrictor reactivity following chronic hypoxia in neonatal rats
Abstract
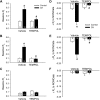
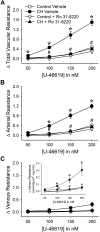
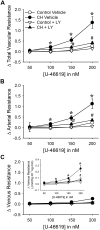
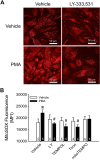
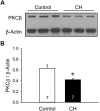
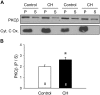
Reactive oxygen species (ROS), mitochondrial dysfunction, and excessive vasoconstriction are important contributors to chronic hypoxia (CH)-induced neonatal pulmonary hypertension. On the basis of evidence that PKCβ and mitochondrial oxidative stress are involved in several cardiovascular and metabolic disorders, we hypothesized that PKCβ and mitochondrial ROS (mitoROS) signaling contribute to enhanced pulmonary vasoconstriction in neonatal rats exposed to CH. To test this hypothesis, we examined effects of the PKCβ inhibitor LY-333,531, the ROS scavenger 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL), and the mitochondrial antioxidants mitoquinone mesylate (MitoQ) and (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (MitoTEMPO) on vasoconstrictor responses in saline-perfused lungs (in situ) or pressurized pulmonary arteries from 2-wk-old control and CH (12-day exposure, 0.5 atm) rats. Lungs from CH rats exhibited greater basal tone and vasoconstrictor sensitivity to 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U-46619). LY-333,531 and TEMPOL attenuated these effects of CH, while having no effect in lungs from control animals. Basal tone was similarly elevated in isolated pulmonary arteries from neonatal CH rats compared with control rats, which was inhibited by both LY-333,531 and mitochondria-targeted antioxidants. Additional experiments assessing mitoROS generation with the mitochondria-targeted ROS indicator MitoSOX revealed that a PKCβ-mitochondrial oxidant signaling pathway can be pharmacologically stimulated by the PKC activator phorbol 12-myristate 13-acetate in primary cultures of pulmonary artery smooth muscle cells (PASMCs) from control neonates. Finally, we found that neonatal CH increased mitochondrially localized PKCβ in pulmonary arteries as assessed by Western blotting of subcellular fractions. We conclude that PKCβ activation leads to mitoROS production in PASMCs from neonatal rats. Furthermore, this signaling axis may account for enhanced pulmonary vasoconstrictor sensitivity following CH exposure.NEW & NOTEWORTHY This research demonstrates a novel contribution of PKCβ and mitochondrial reactive oxygen species signaling to increased pulmonary vasoconstrictor reactivity in chronically hypoxic neonates. The results provide a potential mechanism by which chronic hypoxia increases both basal and agonist-induced pulmonary arterial smooth muscle tone, which may contribute to neonatal pulmonary hypertension.
Keywords: PKCβ; mitochondria; oxidative stress; pulmonary hypertension; vascular disease.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society . Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 132: 2037–2099, 2015. [Erratum in Circulation 133: e368, 2016.] doi: 10.1161/CIR.0000000000000329. - DOI - PubMed
-
- Afolayan AJ, Eis A, Alexander M, Michalkiewicz T, Teng RJ, Lakshminrusimha S, Konduri GG. Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L40–L49, 2016. doi: 10.1152/ajplung.00392.2014. - DOI - PMC - PubMed
-
- Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, Konduri GG. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 303: L870–L879, 2012. doi: 10.1152/ajplung.00098.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

