Subacute TGFβ Exposure Drives Airway Hyperresponsiveness in Cystic Fibrosis Mice through the PI3K Pathway
- PMID: 31922900
- PMCID: PMC7193791
- DOI: 10.1165/rcmb.2019-0158OC
Subacute TGFβ Exposure Drives Airway Hyperresponsiveness in Cystic Fibrosis Mice through the PI3K Pathway
Abstract
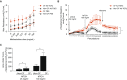
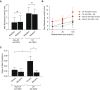
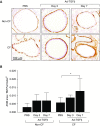
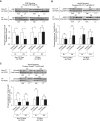
Cystic fibrosis (CF) is a lethal genetic disease characterized by progressive lung damage and airway obstruction. The majority of patients demonstrate airway hyperresponsiveness (AHR), which is associated with more rapid lung function decline. Recent studies in the neonatal CF pig demonstrated airway smooth muscle (ASM) dysfunction. These findings, combined with observed CF transmembrane conductance regulator (CFTR) expression in ASM, suggest that a fundamental defect in ASM function contributes to lung function decline in CF. One established driver of AHR and ASM dysfunction is transforming growth factor (TGF) β1, a genetic modifier of CF lung disease. Prior studies demonstrated that TGFβ exposure in CF mice drives features of CF lung disease, including goblet cell hyperplasia and abnormal lung mechanics. CF mice displayed aberrant responses to pulmonary TGFβ, with elevated PI3K signaling and greater increases in lung resistance compared with controls. Here, we show that TGFβ drives abnormalities in CF ASM structure and function through PI3K signaling that is enhanced in CFTR-deficient lungs. CF and non-CF mice were exposed intratracheally to an adenoviral vector containing the TGFβ1 cDNA, empty vector, or PBS only. We assessed methacholine-induced AHR, bronchodilator response, and ASM area in control and CF mice. Notably, CF mice demonstrated enhanced AHR and bronchodilator response with greater ASM area increases compared with non-CF mice. Furthermore, therapeutic inhibition of PI3K signaling mitigated the TGFβ-induced AHR and goblet cell hyperplasia in CF mice. These results highlight a latent AHR phenotype in CFTR deficiency that is enhanced through TGFβ-induced PI3K signaling.
Keywords: CFTR; airway hyperresponsiveness; airway smooth muscle; cystic fibrosis; transforming growth factor β.
Figures

Comment in
-
Is PI3K a Villain in Cystic Fibrosis?Am J Respir Cell Mol Biol. 2020 May;62(5):552-553. doi: 10.1165/rcmb.2020-0029ED. Am J Respir Cell Mol Biol. 2020. PMID: 32011906 Free PMC article. No abstract available.
Similar articles
-
CFTR dysfunction in smooth muscle drives TGFβ dependent airway hyperreactivity.Respir Res. 2023 Aug 11;24(1):198. doi: 10.1186/s12931-023-02495-2. Respir Res. 2023. PMID: 37568151 Free PMC article.
-
Subacute TGFβ expression drives inflammation, goblet cell hyperplasia, and pulmonary function abnormalities in mice with effects dependent on CFTR function.Am J Physiol Lung Cell Mol Physiol. 2018 Sep 1;315(3):L456-L465. doi: 10.1152/ajplung.00530.2017. Epub 2018 Jun 7. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29877096 Free PMC article.
-
Contractile Properties of Intrapulmonary Airway Smooth Muscle in Cystic Fibrosis.Am J Respir Cell Mol Biol. 2019 Apr;60(4):434-444. doi: 10.1165/rcmb.2018-0005OC. Am J Respir Cell Mol Biol. 2019. PMID: 30359078
-
TGFβ as a therapeutic target in cystic fibrosis.Expert Opin Ther Targets. 2018 Feb;22(2):177-189. doi: 10.1080/14728222.2018.1406922. Epub 2017 Dec 13. Expert Opin Ther Targets. 2018. PMID: 29168406 Free PMC article. Review.
-
The contribution of airway smooth muscle to airway narrowing and airway hyperresponsiveness in disease.Eur Respir J. 2000 Aug;16(2):349-54. doi: 10.1034/j.1399-3003.2000.16b25.x. Eur Respir J. 2000. PMID: 10968513 Review.
Cited by
-
Recruited monocytes/macrophages drive pulmonary neutrophilic inflammation and irreversible lung tissue remodeling in cystic fibrosis.Cell Rep. 2022 Dec 13;41(11):111797. doi: 10.1016/j.celrep.2022.111797. Cell Rep. 2022. PMID: 36516754 Free PMC article.
-
Alpha-1 antitrypsin limits neutrophil extracellular trap disruption of airway epithelial barrier function.Front Immunol. 2023 Jan 10;13:1023553. doi: 10.3389/fimmu.2022.1023553. eCollection 2022. Front Immunol. 2023. PMID: 36703990 Free PMC article.
-
CFTR dysfunction in smooth muscle drives TGFβ dependent airway hyperreactivity.Respir Res. 2023 Aug 11;24(1):198. doi: 10.1186/s12931-023-02495-2. Respir Res. 2023. PMID: 37568151 Free PMC article.
-
Is PI3K a Villain in Cystic Fibrosis?Am J Respir Cell Mol Biol. 2020 May;62(5):552-553. doi: 10.1165/rcmb.2020-0029ED. Am J Respir Cell Mol Biol. 2020. PMID: 32011906 Free PMC article. No abstract available.
-
Update in Pediatrics 2020.Am J Respir Crit Care Med. 2021 Aug 1;204(3):274-284. doi: 10.1164/rccm.202103-0605UP. Am J Respir Crit Care Med. 2021. PMID: 34126039 Free PMC article. No abstract available.
References
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. - PubMed
-
- Eggleston PA, Rosenstein BJ, Stackhouse CM, Alexander MF. Airway hyperreactivity in cystic fibrosis: clinical correlates and possible effects on the course of the disease. Chest. 1988;94:360–365. - PubMed
-
- Regamey N, Ochs M, Hilliard TN, Mühlfeld C, Cornish N, Fleming L, et al. Increased airway smooth muscle mass in children with asthma, cystic fibrosis, and non–cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2008;177:837–843. - PubMed
-
- Kent BD, Lane SJ, van Beek EJ, Dodd JD, Costello RW, Tiddens HA. Asthma and cystic fibrosis: a tangled web. Pediatr Pulmonol. 2014;49:205–213. - PubMed
-
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. National Hypertonic Saline in Cystic Fibrosis (NHSCF) Study Group. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

