Prospective Feasibility Trial of a Novel Strategy of Facilitated Cascade Genetic Testing Using Telephone Counseling
- PMID: 31922918
- PMCID: PMC7193751
- DOI: 10.1200/JCO.19.02005
Prospective Feasibility Trial of a Novel Strategy of Facilitated Cascade Genetic Testing Using Telephone Counseling
Abstract
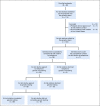
Patients and methods: Probands with newly diagnosed cancer-associated pathogenic variants were offered facilitated cascade testing whereby the genetics team identified and contacted ARRs by telephone to disclose the familial pathogenic variant and offer telephone counseling and mailed saliva testing. Results and guideline-based recommendations were reviewed by telephone and shared with the primary care physician.
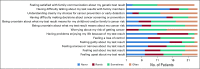
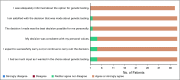
Results: Thirty probands were enrolled, and 114 ARRs were identified. Twelve ARRs were excluded (lived outside of the United States, n = 5; proband did not approve of contact, n = 7). Among 102 ARRs telephoned, contact was established with 95 (93%). Among 114 identified ARRs, 66 (58%) completed genetic testing. Among those completing testing, 27 (41%) carried the familial pathogenic variant. Surveys of ARRs at the time of genetic testing and 6 months later demonstrated low levels of anxiety, depression, distress, and uncertainty and high levels of satisfaction with testing. At 6 months, 7 ARRs with pathogenic variants had undergone cancer surveillance interventions and 4 had undergone cancer risk-reducing surgery.
Conclusion: Facilitated cascade testing with telephone genetic counseling and mailed saliva kits resulted in high testing uptake among ARRs. Positive genetic testing resulted in utilization of genetically targeted primary disease prevention at short-term follow-up. Facilitated cascade testing is a straightforward, low-cost, easily implemented strategy with significant potential to promote early detection for affected ARRs and reduce cancer mortality and should be evaluated in larger scale clinical trials.
Figures

Comment in
-
Emerging Opportunity of Cascade Genetic Testing for Population-Wide Cancer Prevention and Control.J Clin Oncol. 2020 May 1;38(13):1371-1374. doi: 10.1200/JCO.20.00140. Epub 2020 Feb 25. J Clin Oncol. 2020. PMID: 32097078 No abstract available.
-
Peridiagnostic and cascade cancer genetic testing.Nat Rev Clin Oncol. 2020 May;17(5):277-278. doi: 10.1038/s41571-020-0348-4. Nat Rev Clin Oncol. 2020. PMID: 32152486 No abstract available.
References
-
- Committee on Gynecologic Practice ACOG Committee Opinion No. 727: Cascade testing: Testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:e31–e34. - PubMed
-
- Randall LM, Pothuri B, Swisher EM, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology white paper. Gynecol Oncol. 2017;146:217–224. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

