Darunavir Pharmacokinetics With an Increased Dose During Pregnancy
- PMID: 31923087
- PMCID: PMC7258985
- DOI: 10.1097/QAI.0000000000002261
Darunavir Pharmacokinetics With an Increased Dose During Pregnancy
Abstract
Background: This study aims to evaluate the pharmacokinetics of an increased dose of darunavir (800 mg twice daily) with 100 mg ritonavir during pregnancy and postpartum.
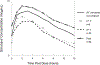
Methods: Darunavir (DRV) and ritonavir (RTV; r) intensive pharmacokinetic evaluations were performed at steady state during the second and third trimesters of pregnancy (DRV/r 800/100 mg bid) and 2-3 weeks postpartum (DRV/r 600/100 mg twice daily). Plasma concentrations of darunavir and ritonavir were measured using high-performance liquid chromatography. Target darunavir area under the concentration time curve (AUC) was >70% (43.6 μg × h/mL) of median AUC (62.3 μg × h/mL) in nonpregnant adults on twice daily darunavir-ritonavir 600/100 mg.
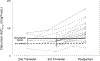
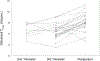
Results: Twenty-four women were included in the analysis. Darunavir AUC0-12 was lower with the increased dose during the second {[geometric mean ratio (GMR) of 0.62 (IQR 0.44-0.88); P = 0.055]} and third trimesters [GMR 0.64 (IQR 0.55-0.73); P = <0.001] compared with postpartum. Darunavir apparent clearance was higher during the second [GMR 1.77 (IQR 1.24-2.51); P = 0.039] and third trimesters [GMR 2.01 (IQR 1.17-2.35); P = <0.001] compared with postpartum. Similarly, ritonavir AUC0-12 was lower during the third trimester [GMR 0.65 (IQR 0.52-0.82); P = 0.007] compared with postpartum, whereas its apparent clearance was higher during the third trimester [GMR 1.53 (IQR 1.22-1.92); P = 0.008] compared with postpartum. No major drug-related safety concerns were noted.
Conclusions: Increasing darunavir dose to 800 mg BID failed to significantly increase darunavir exposure compared with 600 mg BID. Other strategies, such as increasing the ritonavir dose should be investigated.
Figures


References
-
- Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Transmission in the United States. 2018; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf Accessed Accessed 1/11/19.
-
- Crauwels HM, Kakuda TN, Ryan B, et al. Pharmacokinetics of once-daily darunavir/ritonavir in HIV-1-infected pregnant women. HIV Med. 2016;17(9):643–652. - PubMed
-
- Colbers A, Molto J, Ivanovic J, et al. Pharmacokinetics of total and unbound darunavir in HIV-1-infected pregnant women. J Antimicrob Chemother. 2015;70(2):534–542. - PubMed

