A Pathogen-Responsive Gene Cluster for Highly Modified Fatty Acids in Tomato
- PMID: 31923394
- PMCID: PMC6956849
- DOI: 10.1016/j.cell.2019.11.037
A Pathogen-Responsive Gene Cluster for Highly Modified Fatty Acids in Tomato
Abstract
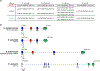
In response to biotic stress, plants produce suites of highly modified fatty acids that bear unusual chemical functionalities. Despite their chemical complexity and proposed roles in pathogen defense, little is known about the biosynthesis of decorated fatty acids in plants. Falcarindiol is a prototypical acetylenic lipid present in carrot, tomato, and celery that inhibits growth of fungi and human cancer cell lines. Using a combination of untargeted metabolomics and RNA sequencing, we discovered a biosynthetic gene cluster in tomato (Solanum lycopersicum) required for falcarindiol production. By reconstituting initial biosynthetic steps in a heterologous host and generating transgenic pathway mutants in tomato, we demonstrate a direct role of the cluster in falcarindiol biosynthesis and resistance to fungal and bacterial pathogens in tomato leaves. This work reveals a mechanism by which plants sculpt their lipid pool in response to pathogens and provides critical insight into the complex biochemistry of alkynyl lipid production.
Keywords: acetylenase; acetylenic lipids; antifungal oxylipin; falcarindiol; plant lipid gene cluster; plant natural product biosynthesis; transcriptomics; untargeted metabolomics.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


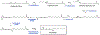
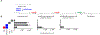


References
-
- Abramovitch R, and Martin G (2005). AvrPtoB: a bacterial type III effector that both elicits and suppresses programmed cell death associated with plant immunity. FEMS Microbiology Letters 245, 1–8. - PubMed
-
- Bentley RK, Bhattacharjee D, Jones ERH, and Thaller V (1969). Natural acetylenes. Part XXVIII. C17-polyacetylenic alcohols from the Umbellifer Daucus carota L. (carrot): alkylation of benzene by acetylenyl(vinyl)carbinols in the presence of toluene-p-sulphonic acid. J. Chem. Soc., C 685–688.
-
- Bernard A, Domergue F, Pascal S, Jetter R, Renne C, Faure JD, Haslam RP, Napier JA, Lessire R, and Joubes J (2012). Reconstitution of Plant Alkane Biosynthesis in Yeast Demonstrates That Arabidopsis ECERIFERUM1 and ECERIFERUM3 Are Core Components of a Very-Long-Chain Alkane Synthesis Complex. Plant Cell 24, 3106–3118. - PMC - PubMed
-
- Blée E (2002). Impact of phyto-oxylipins in plant defense. Trends in Plant Science 7, 315–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

