Treatment outcomes of patients with chronic hepatitis C receiving sofosbuvir-based combination therapy within national hepatitis C elimination program in the country of Georgia
- PMID: 31924172
- PMCID: PMC6954615
- DOI: 10.1186/s12879-019-4741-5
Treatment outcomes of patients with chronic hepatitis C receiving sofosbuvir-based combination therapy within national hepatitis C elimination program in the country of Georgia
Abstract
Background: Georgia has one of the highest HCV prevalence in the world and launched the world's first national HCV elimination programs in 2015. Georgia set the ambitious target of diagnosing 90% of people living with HCV, treating 95% of those diagnosed and curing 95% of treated patients by 2020. We report outcomes of Sofosbuvir (SOF) based treatment regimens in patients with chronic HCV infection in Georgia.
Methods: Patients with cirrhosis, advanced liver fibrosis and severe extrahepatic manifestations were enrolled in the treatment program. Initial treatment consisted of SOF plus ribavirin (RBV) with or without pegylated interferon (INF). Sustained virologic response (SVR) was defined as undetectable HCV RNA at least 12 weeks after the end of treatment. SVR were calculated using both per-protocol and modified intent-to-treat (mITT) analysis. Results for patients who completed treatment through 31 October 2018 were analyzed.
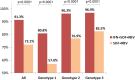
Results: Of the 7342 patients who initiated treatment with SOF-based regimens, 5079 patients were tested for SVR. Total SVR rate was 82.1% in per-protocol analysis and 74.5% in mITT analysis. The lowest response rate was observed among genotype 1 patients (69.5%), intermediate response rate was achieved in genotype 2 patients (81.4%), while the highest response rate was among genotype 3 patients (91.8%). Overall, SOF/RBV regimens achieved lower response rates than IFN/SOF/RBV regimen (72.1% vs 91.3%, P < 0.0001). In multivariate analysis being infected with HCV genotype 2 (RR =1.10, CI [1.05-1.15]) and genotype 3 (RR = 1.14, CI [1.11-1.18]) were associated with higher SVR. Patients with cirrhosis (RR = 0.95, CI [0.93-0.98]), receiving treatment regimens of SOF/RBV 12 weeks, SOF/RBV 20 weeks, SOF/RBV 24 weeks and SOF/RBV 48 weeks (RR = 0.85, CI [0.81-0.91]; RR = 0.86, CI [0.82-0.92]; RR = 0.88, CI [0.85-0.91] and RR = 0.92, CI [0.87-0.98], respectively) were less likely to achieve SVR.
Conclusions: Georgia's real world experience resulted in high overall response rates given that most patients had severe liver damage. Our results provide clear evidence that SOF plus IFN and RBV for 12 weeks can be considered a treatment option for eligible patients with all three HCV genotypes. With introduction of next generation DAAs, significantly improved response rates are expected, paving the way for Georgia to achieve HCV elimination goals.
Keywords: DAAs; Elimination; Georgia; HCV; SVR.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3.J Hepatol. 2019 Jan;70(1):15-23. doi: 10.1016/j.jhep.2018.09.018. Epub 2018 Sep 26. J Hepatol. 2019. PMID: 30266283
-
Real-world effectiveness of direct-acting antiviral agents for chronic hepatitis C patients with genotype-2 infection after completed treatment.Kaohsiung J Med Sci. 2021 Apr;37(4):334-345. doi: 10.1002/kjm2.12315. Epub 2020 Nov 5. Kaohsiung J Med Sci. 2021. PMID: 33151016 Free PMC article.
-
Efficacy and Safety of Ledipasvir/Sofosbuvir with and without Ribavirin in Patients with Chronic Hepatitis C Virus Genotype 1 Infection: a meta-analysis.Int J Infect Dis. 2017 Feb;55:56-71. doi: 10.1016/j.ijid.2016.12.023. Epub 2016 Dec 29. Int J Infect Dis. 2017. PMID: 28040553
-
Combination of sofosbuvir, pegylated-interferon and ribavirin for treatment of hepatitis C virus genotype 1 infection: a systematic review and meta-analysis.Daru. 2017 Apr 20;25(1):11. doi: 10.1186/s40199-017-0177-x. Daru. 2017. PMID: 28427463 Free PMC article.
-
Treatment of hepatitis C virus genotype 3 infection with direct-acting antiviral agents.Braz J Med Biol Res. 2016 Oct 24;49(11):e5504. doi: 10.1590/1414-431X20165504. Braz J Med Biol Res. 2016. PMID: 27783808 Free PMC article. Review.
Cited by
-
Economic evaluation of the Hepatitis C virus elimination program in the country of Georgia, 2015 to 2017.Liver Int. 2023 Mar;43(3):558-568. doi: 10.1111/liv.15431. Epub 2022 Oct 4. Liver Int. 2023. PMID: 36129625 Free PMC article.
-
Training the healthcare workforce: the global experience with telementorship for hepatitis B and hepatitis C.BMC Health Serv Res. 2023 Aug 2;23(1):824. doi: 10.1186/s12913-023-09849-y. BMC Health Serv Res. 2023. PMID: 37533025 Free PMC article.
-
Pre-Exposure Prophylaxis for viral infections other than HIV.Infez Med. 2022 Sep 1;30(3):362-371. doi: 10.53854/liim-3003-5. eCollection 2022. Infez Med. 2022. PMID: 36148176 Free PMC article. Review.
-
Prevalence of Toxoplasma gondii 36-KDa antigen and chronic Hepatitis C: another evidence of an Association.J Parasit Dis. 2021 Dec;45(4):1049-1054. doi: 10.1007/s12639-021-01351-8. Epub 2021 May 6. J Parasit Dis. 2021. PMID: 34789988 Free PMC article.
-
Treatment outcomes of chronic liver disease and associated factors among patients treated at hospitals in Bahir Dar city, north-west Ethiopia.BMC Gastroenterol. 2025 Mar 6;25(1):141. doi: 10.1186/s12876-025-03719-z. BMC Gastroenterol. 2025. PMID: 40050738 Free PMC article.
References
-
- WHO Hepatitis C fact sheet. Updated April 2017. http://www.who.int/mediacentre/factsheets/fs164/en/. .
-
- Kowdley KV, Lawitz E, Crespo I, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381(9883):2100–2107. doi: 10.1016/S0140-6736(13)60247-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources