Precision cut lung slices: a novel versatile tool to examine host-pathogen interaction in the chicken lung
- PMID: 31924278
- PMCID: PMC6954617
- DOI: 10.1186/s13567-019-0733-0
Precision cut lung slices: a novel versatile tool to examine host-pathogen interaction in the chicken lung
Abstract
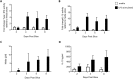
The avian respiratory tract is a common entry route for many pathogens and an important delivery route for vaccination in the poultry industry. Immune responses in the avian lung have mostly been studied in vivo due to the lack of robust, relevant in vitro and ex vivo models mimicking the microenvironment. Precision-cut lung slices (PCLS) have the major advantages of maintaining the 3-dimensional architecture of the lung and includes heterogeneous cell populations. PCLS have been obtained from a number of mammalian species and from chicken embryos. However, as the embryonic lung is physiologically undifferentiated and immunologically immature, it is less suitable to examine complex host-pathogen interactions including antimicrobial responses. Here we prepared PCLS from immunologically mature chicken lungs, tested different culture conditions, and found that serum supplementation has a detrimental effect on the quality of PCLS. Viable cells in PCLS remained present for ≥ 40 days, as determined by viability assays and sustained motility of fluorescent mononuclear phagocytic cells. The PCLS were responsive to lipopolysaccharide stimulation, which induced the release of nitric oxide, IL-1β, type I interferons and IL-10. Mononuclear phagocytes within the tissue maintained phagocytic activity, with live cell imaging capturing interactions with latex beads and an avian pathogenic Escherichia coli strain. Finally, the PCLS were also shown to be permissive to infection with low pathogenic avian influenza viruses. Taken together, immunologically mature chicken PCLS provide a suitable model to simulate live organ responsiveness and cell dynamics, which can be readily exploited to examine host-pathogen interactions and inflammatory responses.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




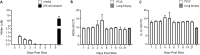

References
-
- Siminski JT, Kavanagh TJ, Chi E, Raghu G. Long-term maintenance of mature pulmonary parenchyma cultured in serum-free conditions. Am J Physiol. 1992;262:L105–110. - PubMed
-
- Rosales Gerpe MC, van Vloten JP, Santry LA, de Jong J, Mould RC, Pelin A, Bell JC, Bridle BW, Wootton SK. Use of precision-cut lung slices as an ex vivo tool for evaluating viruses and viral vectors for gene and oncolytic therapy. Mol Ther Methods Clin Dev. 2018;10:245–256. doi: 10.1016/j.omtm.2018.07.010. - DOI - PMC - PubMed
-
- Meng F, Wu NH, Nerlich A, Herrler G, Valentin-Weigand P, Seitz M. Dynamic virus-bacterium interactions in a porcine precision-cut lung slice coinfection model: swine influenza virus paves the way for Streptococcus suis infection in a two-step process. Infect Immun. 2015;83:2806–2815. doi: 10.1128/IAI.00171-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/M028305/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004219/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 2015-67015-23093/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M028208/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/20002172/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

