Vanillin Production in Pseudomonas: Whole-Genome Sequencing of Pseudomonas sp. Strain 9.1 and Reannotation of Pseudomonas putida CalA as a Vanillin Reductase
- PMID: 31924622
- PMCID: PMC7054097
- DOI: 10.1128/AEM.02442-19
Vanillin Production in Pseudomonas: Whole-Genome Sequencing of Pseudomonas sp. Strain 9.1 and Reannotation of Pseudomonas putida CalA as a Vanillin Reductase
Abstract
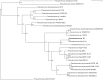
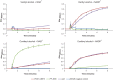
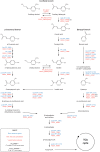
Microbial degradation of lignin and its related aromatic compounds has great potential for the sustainable production of chemicals and bioremediation of contaminated soils. We previously isolated Pseudomonas sp. strain 9.1 from historical waste deposits (forming so-called fiber banks) released from pulp and paper mills along the Baltic Sea coast. The strain accumulated vanillyl alcohol during growth on vanillin, and while reported in other microbes, this phenotype is less common in wild-type pseudomonads. As the reduction of vanillin to vanillyl alcohol is an undesired trait in Pseudomonas strains engineered to accumulate vanillin, connecting the strain 9.1 phenotype with a genotype would increase the fundamental understanding and genetic engineering potential of microbial vanillin metabolism. The genome of Pseudomonas sp. 9.1 was sequenced and assembled. Annotation identified oxidoreductases with homology to Saccharomyces cerevisiae alcohol dehydrogenase ScADH6p, known to reduce vanillin to vanillyl alcohol, in both the 9.1 genome and the model strain Pseudomonas putida KT2440. Recombinant expression of the Pseudomonas sp. 9.1 FEZ21_09870 and P. putida KT2440 PP_2426 (calA) genes in Escherichia coli revealed that these open reading frames encode aldehyde reductases that convert vanillin to vanillyl alcohol, and that P. putida KT2440 PP_3839 encodes a coniferyl alcohol dehydrogenase that oxidizes coniferyl alcohol to coniferyl aldehyde (i.e., the function previously assigned to calA). The deletion of PP_2426 in P. putida GN442 engineered to accumulate vanillin resulted in a decrease in by-product (vanillyl alcohol) yield from 17% to ∼1%. Based on these results, we propose the reannotation of PP_2426 and FEZ21_09870 as areA and PP_3839 as calA-IIIMPORTANCE Valorization of lignocellulose (nonedible plant matter) is of key interest for the sustainable production of chemicals from renewable resources. Lignin, one of the main constituents of lignocellulose, is a heterogeneous aromatic biopolymer that can be chemically depolymerized into a heterogeneous mixture of aromatic building blocks; those can be further converted by certain microbes into value-added aromatic chemicals, e.g., the flavoring agent vanillin. We previously isolated a Pseudomonas sp. strain with the (for the genus) unusual trait of vanillyl alcohol production during growth on vanillin. Whole-genome sequencing of the isolate led to the identification of a vanillin reductase candidate gene whose deletion in a recombinant vanillin-accumulating P. putida strain almost completely alleviated the undesired vanillyl alcohol by-product yield. These results represent an important step toward biotechnological production of vanillin from lignin using bacterial cell factories.
Keywords: NAD(P)H-dependent oxidoreductases; Pseudomonas; calA; de novo assembly; gene reannotation; vanillyl alcohol.
Copyright © 2020 García-Hidalgo et al.
Figures



Similar articles
-
Comprehensive proteome analysis of the response of Pseudomonas putida KT2440 to the flavor compound vanillin.J Proteomics. 2014 Sep 23;109:212-27. doi: 10.1016/j.jprot.2014.07.006. Epub 2014 Jul 12. J Proteomics. 2014. PMID: 25026441
-
Identification of the Gene Responsible for Lignin-Derived Low-Molecular-Weight Compound Catabolism in Pseudomonas sp. Strain LLC-1.Genes (Basel). 2020 Nov 27;11(12):1416. doi: 10.3390/genes11121416. Genes (Basel). 2020. PMID: 33260964 Free PMC article.
-
Genetic engineering of Pseudomonas putida KT2440 for rapid and high-yield production of vanillin from ferulic acid.Appl Microbiol Biotechnol. 2014 Jan;98(1):137-49. doi: 10.1007/s00253-013-5303-1. Epub 2013 Oct 18. Appl Microbiol Biotechnol. 2014. PMID: 24136472
-
Pseudomonas putida as a functional chassis for industrial biocatalysis: From native biochemistry to trans-metabolism.Metab Eng. 2018 Nov;50:142-155. doi: 10.1016/j.ymben.2018.05.005. Epub 2018 May 16. Metab Eng. 2018. PMID: 29758287 Review.
-
Pseudomonas putida KT2440: the long journey of a soil-dweller to become a synthetic biology chassis.J Bacteriol. 2024 Jul 25;206(7):e0013624. doi: 10.1128/jb.00136-24. Epub 2024 Jul 8. J Bacteriol. 2024. PMID: 38975763 Free PMC article. Review.
Cited by
-
Engineering a vanillate-producing strain of Pseudomonas sp. NGC7 corresponding to aromatic compounds derived from the continuous catalytic alkaline oxidation of sulfite lignin.Microb Cell Fact. 2024 Nov 19;23(1):313. doi: 10.1186/s12934-024-02590-z. Microb Cell Fact. 2024. PMID: 39563320 Free PMC article.
-
Production of Vanillin From Ferulic Acid by Pseudomonas putida KT2440 Using Metabolic Engineering and In Situ Product Recovery.Microb Biotechnol. 2025 May;18(5):e70152. doi: 10.1111/1751-7915.70152. Microb Biotechnol. 2025. PMID: 40410945 Free PMC article.
-
Metabolic engineering of Pseudomonas putida for production of vanillylamine from lignin-derived substrates.Microb Biotechnol. 2021 Nov;14(6):2448-2462. doi: 10.1111/1751-7915.13764. Epub 2021 Feb 3. Microb Biotechnol. 2021. PMID: 33533574 Free PMC article.
-
Engineering a Cytochrome P450 for Demethylation of Lignin-Derived Aromatic Aldehydes.JACS Au. 2021 Feb 4;1(3):252-261. doi: 10.1021/jacsau.0c00103. eCollection 2021 Mar 22. JACS Au. 2021. PMID: 34467290 Free PMC article.
-
Resolving the metabolism of monolignols and other lignin-related aromatic compounds in Xanthomonas citri.Nat Commun. 2024 Sep 12;15(1):7994. doi: 10.1038/s41467-024-52367-6. Nat Commun. 2024. PMID: 39266555 Free PMC article.
References
-
- Holladay JE, White JF, Bozell JJ, Johnson D. 2007. Top value added chemicals from biomass. Volume II: results of screening for potential candidates from biorefinery lignin. Pacific Northwest National Lab (PNNL), Richland, WA: https://www.pnnl.gov/main/publications/external/technical_reports/PNNL-1....
-
- Ayyachamy M, Cliffe FE, Coyne JM, Collier J, Tuohy MG. 2013. Lignin: untapped biopolymers in biomass conversion technologies. Biomass Conv Bioref 3:255–269. doi:10.1007/s13399-013-0084-4. - DOI
-
- Stewart D. 2008. Lignin as a base material for materials applications: chemistry, application and economics. Ind Crops Prod 27:202–207. doi:10.1016/j.indcrop.2007.07.008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

