Loss of PARP-1 attenuates diabetic arteriosclerotic calcification via Stat1/Runx2 axis
- PMID: 31924749
- PMCID: PMC6954221
- DOI: 10.1038/s41419-019-2215-8
Loss of PARP-1 attenuates diabetic arteriosclerotic calcification via Stat1/Runx2 axis
Erratum in
-
Correction: Loss of PARP-1 attenuates diabetic arteriosclerotic calcification via Stat1/Runx2 axis.Cell Death Dis. 2020 Feb 6;11(2):97. doi: 10.1038/s41419-020-2292-8. Cell Death Dis. 2020. PMID: 32029701 Free PMC article.
Abstract
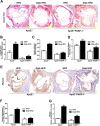
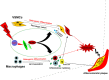
Accelerated atherosclerotic calcification is responsible for plaque burden, especially in diabetes. The regulatory mechanism for atherosclerotic calcification in diabetes is poorly characterized. Here we show that deletion of PARP-1, a main enzyme in diverse metabolic complications, attenuates diabetic atherosclerotic calcification and decreases vessel stiffening in mice through Runx2 suppression. Specifically, PARP-1 deficiency reduces diabetic arteriosclerotic calcification by regulating Stat1-mediated synthetic phenotype switching of vascular smooth muscle cells and macrophage polarization. Meanwhile, both vascular smooth muscle cells and macrophages manifested osteogenic differentiation in osteogenic media, which was attenuated by PARP-1/Stat1 inhibition. Notably, Stat1 acts as a positive transcription factor by directly binding to the promoter of Runx2 and promoting atherosclerotic calcification in diabetes. Our results identify a new function of PARP-1, in which metabolism disturbance-related stimuli activate the Runx2 expression mediated by Stat1 transcription to facilitate diabetic arteriosclerotic calcification. PARP-1 inhibition may therefore represent a useful therapy for this challenging complication.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Diabetic Vascular Calcification Mediated by the Collagen Receptor Discoidin Domain Receptor 1 via the Phosphoinositide 3-Kinase/Akt/Runt-Related Transcription Factor 2 Signaling Axis.Arterioscler Thromb Vasc Biol. 2018 Aug;38(8):1878-1889. doi: 10.1161/ATVBAHA.118.311238. Arterioscler Thromb Vasc Biol. 2018. PMID: 29930002 Free PMC article.
-
ILF3 is responsible for hyperlipidemia-induced arteriosclerotic calcification by mediating BMP2 and STAT1 transcription.J Mol Cell Cardiol. 2021 Dec;161:39-52. doi: 10.1016/j.yjmcc.2021.07.011. Epub 2021 Jul 31. J Mol Cell Cardiol. 2021. PMID: 34343541
-
Inhibition of FOXO1/3 promotes vascular calcification.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):175-83. doi: 10.1161/ATVBAHA.114.304786. Epub 2014 Nov 6. Arterioscler Thromb Vasc Biol. 2015. PMID: 25378413 Free PMC article.
-
Transcriptional Programming in Arteriosclerotic Disease: A Multifaceted Function of the Runx2 (Runt-Related Transcription Factor 2).Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):20-34. doi: 10.1161/ATVBAHA.120.313791. Epub 2020 Oct 29. Arterioscler Thromb Vasc Biol. 2021. PMID: 33115268 Free PMC article. Review.
-
Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
Cited by
-
Loss of myeloid Tsc2 predisposes to angiotensin II-induced aortic aneurysm formation in mice.Cell Death Dis. 2022 Nov 18;13(11):972. doi: 10.1038/s41419-022-05423-2. Cell Death Dis. 2022. PMID: 36400753 Free PMC article.
-
Role of Macrophages in the Progression and Regression of Vascular Calcification.Front Pharmacol. 2020 May 8;11:661. doi: 10.3389/fphar.2020.00661. eCollection 2020. Front Pharmacol. 2020. PMID: 32457633 Free PMC article. Review.
-
RAGE Differentially Altered in vitro Responses in Vascular Smooth Muscle Cells and Adventitial Fibroblasts in Diabetes-Induced Vascular Calcification.Front Physiol. 2021 Jun 7;12:676727. doi: 10.3389/fphys.2021.676727. eCollection 2021. Front Physiol. 2021. PMID: 34163373 Free PMC article.
-
Transcription factors: key regulatory targets of vascular smooth muscle cell in atherosclerosis.Mol Med. 2023 Jan 5;29(1):2. doi: 10.1186/s10020-022-00586-2. Mol Med. 2023. PMID: 36604627 Free PMC article. Review.
-
Naringenin suppresses BEAS-2B-derived extracellular vesicular cargoes disorder caused by cigarette smoke extract thereby inhibiting M1 macrophage polarization.Front Immunol. 2022 Jul 18;13:930476. doi: 10.3389/fimmu.2022.930476. eCollection 2022. Front Immunol. 2022. PMID: 35924248 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

