Piperlongumine regulates epigenetic modulation and alleviates psoriasis-like skin inflammation via inhibition of hyperproliferation and inflammation
- PMID: 31924750
- PMCID: PMC6954241
- DOI: 10.1038/s41419-019-2212-y
Piperlongumine regulates epigenetic modulation and alleviates psoriasis-like skin inflammation via inhibition of hyperproliferation and inflammation
Abstract
Psoriasis is an autoimmune skin disease, where chronic immune responses due to exaggerated cytokine signaling, abnormal differentiation, and evasion of keratinocytes apoptosis plays a crucial role in mediating abnormal keratinocytes hyperproliferation. From the therapeutic perspective, the molecules with strong anti-proliferative and anti-inflammatory properties could have tremendous relevance. In this study, we demonstrated that piperlongumine (PPL) treatment effectively abrogated the hyperproliferation and differentiation of keratinocytes by inducing ROS-mediated late apoptosis with loss of mitochondrial membrane potential. Besides, the arrest of cell cycle was found at Sub-G1 phase as a result of DNA fragmentation. Molecularly, inhibition of STAT3 and Akt signaling was observed with a decrease in proliferative markers such as PCNA, ki67, and Cyclin D1 along with anti-apoptotic Bcl-2 protein expression. Keratin 17 is a critical regulator of keratinocyte differentiation, and it was found to be downregulated with PPL significantly. Furthermore, prominent anti-inflammatory effects were observed by inhibition of lipopolysaccharide (LPS)/Imiquimod (IMQ)-induced p65 NF-κB signaling cascade and strongly inhibited the production of cytokine storm involved in psoriasis-like skin inflammation, thus led to the restoration of normal epidermal architecture with reduction of epidermal hyperplasia and splenomegaly. In addition, PPL epigenetically inhibited histone-modifying enzymes, which include histone deacetylases (HDACs) of class I (HDAC1-4) and class II (HDAC6) evaluated by immunoblotting and HDAC enzyme assay kit. In addition, our results show that PPL effectively inhibits the nuclear translocation of p65 and a histone modulator HDAC3, thus sequestered in the cytoplasm of macrophages. Furthermore, PPL effectively enhanced the protein-protein interactions of HDAC3 and p65 with IκBα, which was disrupted by LPS stimulation and were evaluated by Co-IP and molecular modeling. Collectively, our findings indicate that piperlongumine may serve as an anti-proliferative and anti-inflammatory agent and could serve as a potential therapeutic option in treating psoriasis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

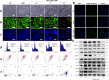
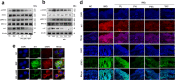
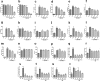
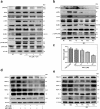


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

