First-in-human clinical trial to assess the safety, tolerability and pharmacokinetics of P218, a novel candidate for malaria chemoprotection
- PMID: 31925817
- PMCID: PMC7256114
- DOI: 10.1111/bcp.14219
First-in-human clinical trial to assess the safety, tolerability and pharmacokinetics of P218, a novel candidate for malaria chemoprotection
Abstract
Aims: This first-in-human clinical trial of P218, a novel dihydrofolate reductase inhibitor antimalarial candidate, assessed safety, tolerability, pharmacokinetics and food effects in healthy subjects.
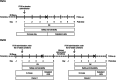
Methods: The study consisted of two parts. Part A was a double-blind, randomized, placebo-controlled, parallel group, ascending dose study comprising seven fasted cohorts. Eight subjects/cohort were randomized (3:1) to receive either a single oral dose of P218 (10, 30, 100, 250, 500, 750 and 1000 mg) or placebo. Part B was an open-label, cross-over, fed/fasted cohort (eight subjects) that received a 250 mg single dose of P218 in two treatment periods.
Results: P218 was generally well tolerated across all doses; 21 treatment-emergent adverse events occurred in 15/64 subjects. Nine adverse events in five subjects, all of mild intensity, were judged drug related. No clinically relevant abnormalities in ECG, vital signs or laboratory tests changes were observed. P218 was rapidly absorbed, with Cmax achieved between 0.5 and 2 hours post dose. Plasma concentrations declined bi-exponentially with half-life values ranging from 3.1 to 6.7 hours (10 and 30 mg), increasing up to 8.9 to 19.6 hours (doses up to 1000 mg). Exposure values increased dose-proportionally between 100 and 1000 mg for P218 (parent) and three primary metabolites (P218 β-acyl glucuronide, P218-OH and P218-OH β-acyl glucuronide). Co-administration of P218 with food reduced Cmax by 35% and delayed absorption by 1 hour, with no significant impact on AUC.
Conclusion: P218 displayed favourable safety, tolerability and pharmacokinetics. In view of its short half-life, a long-acting formulation will be needed for malaria chemoprotection.
Keywords: P218; chemoprotection; clinical trial; dihydrofolate reductase inhibitor; malaria; pharmacodynamics; pharmacokinetics.
© 2020 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
M.F.C., C.D., M.E.G., J.M. and S.Ch. are current employees of the study sponsor MMV. E.R. was previously employed at MMV when the study was conducted. G.L. and T.H. are paid consultants of MMV, U.L. and S.Co. are employees of Richmond Pharmacology, UK where the study was performed.
Figures


References
-
- World Health Organisation . World malaria report. Geneva: WHO; [Cited 2019 19 July]. Available from: https://www.who.int/malaria/publications/world-malaria-report-2018/en/.
-
- World Health Organization . Intermittent preventive treatment in pregnancy (IPTp). Geneva: WHO; [Cited 2018 30 June]. Available from: http://www.who.int/malaria/areas/preventive_therapies/pregnancy/en/.
-
- World Health Organisation . Malaria prevention works: let's close the gap. Geneva: WHO; [Cited 2018 30 June]. Available from: http://www.who.int/malaria/publications/atoz/malaria-prevention-works/en/.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

