Intravenous Immunomodulatory Nanoparticle Treatment for Traumatic Brain Injury
- PMID: 31925846
- PMCID: PMC7296512
- DOI: 10.1002/ana.25675
Intravenous Immunomodulatory Nanoparticle Treatment for Traumatic Brain Injury
Abstract
Objective: There are currently no definitive disease-modifying therapies for traumatic brain injury (TBI). In this study, we present a strong therapeutic candidate for TBI, immunomodulatory nanoparticles (IMPs), which ablate a specific subset of hematogenous monocytes (hMos). We hypothesized that prevention of infiltration of these cells into brain acutely after TBI would attenuate secondary damage and preserve anatomic and neurologic function.
Methods: IMPs, composed of US Food and Drug Administration-approved 500nm carboxylated-poly(lactic-co-glycolic) acid, were infused intravenously into wild-type C57BL/6 mice following 2 different models of experimental TBI, controlled cortical impact (CCI), and closed head injury (CHI).
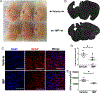
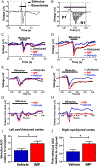
Results: IMP administration resulted in remarkable preservation of both tissue and neurological function in both CCI and CHI TBI models in mice. After acute treatment, there was a reduction in the number of immune cells infiltrating into the brain, mitigation of the inflammatory status of the infiltrating cells, improved electrophysiologic visual function, improved long-term motor behavior, reduced edema formation as assessed by magnetic resonance imaging, and reduced lesion volumes on anatomic examination.
Interpretation: Our findings suggest that IMPs are a clinically translatable acute intervention for TBI with a well-defined mechanism of action and beneficial anatomic and physiologic preservation and recovery. Ann Neurol 2020;87:442-455.
© 2020 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
Nothing to report.
Figures




References
-
- Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. Atlanta, GA: National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention, 2015.
-
- Thurman DJ, Alverson C, Dunn KA, et al. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil 1999;14:602–615. - PubMed
-
- de Souza RS, Pinheiro PP, de Lima Silva JMF, et al. Traumatic brain injury (TBI): morbidity, mortality and economic implications. Int Arch Med 2015. Available at: http://imed.pub/ojs/index.php/iam/article/view/1109. Last accessed on June 6, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

