Differential genetic risk for methamphetamine intake confers differential sensitivity to the temperature-altering effects of other addictive drugs
- PMID: 31925906
- PMCID: PMC7286770
- DOI: 10.1111/gbb.12640
Differential genetic risk for methamphetamine intake confers differential sensitivity to the temperature-altering effects of other addictive drugs
Abstract
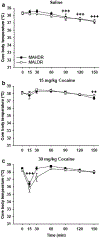
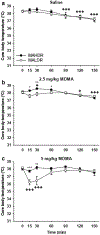
Mice selectively bred for high methamphetamine (MA) drinking (MAHDR), compared with mice bred for low MA drinking (MALDR), exhibit greater sensitivity to MA reward and insensitivity to aversive and hypothermic effects of MA. Previous work identified the trace amine-associated receptor 1 gene (Taar1) as a quantitative trait gene for MA intake that also impacts thermal response to MA. All MAHDR mice are homozygous for the mutant Taar1 m1J allele, whereas all MALDR mice possess at least one copy of the reference Taar1 + allele. To determine if their differential sensitivity to MA-induced hypothermia extends to drugs of similar and different classes, we examined sensitivity to the hypothermic effect of the stimulant cocaine, the amphetamine-like substance 3,4-methylenedioxymethamphetamine (MDMA), and the opioid morphine in these lines. The lines did not differ in thermal response to cocaine, only MALDR mice exhibited a hypothermic response to MDMA, and MAHDR mice were more sensitive to the hypothermic effect of morphine than MALDR mice. We speculated that the μ-opioid receptor gene (Oprm1) impacts morphine response, and genotyped the mice tested for morphine-induced hypothermia. We report genetic linkage between Taar1 and Oprm1; MAHDR mice more often inherit the Oprm1 D2 allele and MALDR mice more often inherit the Oprm1 B6 allele. Data from a family of recombinant inbred mouse strains support the influence of Oprm1 genotype, but not Taar1 genotype, on thermal response to morphine. These results nominate Oprm1 as a genetic risk factor for morphine-induced hypothermia, and provide additional evidence for a connection between drug preference and drug thermal response.
Keywords: addiction; methamphetamine; opioids; thermal regulation; trace amine-associated receptor 1; μ-opioid receptor.
Published 2020. This article is a U.S. Government work and is in the public domain in the USA.
Figures



Similar articles
-
Absence of TAAR1 function increases methamphetamine-induced excitability of dorsal raphe serotonin neurons and drives binge-level methamphetamine intake.Neuropsychopharmacology. 2025 Jun;50(7):1136-1144. doi: 10.1038/s41386-025-02063-w. Epub 2025 Feb 11. Neuropsychopharmacology. 2025. PMID: 39934409 Free PMC article.
-
Opioid sensitivity in mice selectively bred to consume or not consume methamphetamine.Addict Biol. 2014 May;19(3):370-9. doi: 10.1111/adb.12003. Epub 2012 Nov 12. Addict Biol. 2014. PMID: 23145527 Free PMC article.
-
A breeding strategy to identify modifiers of high genetic risk for methamphetamine intake.Genes Brain Behav. 2021 Feb;20(2):e12667. doi: 10.1111/gbb.12667. Epub 2020 Jun 17. Genes Brain Behav. 2021. PMID: 32424970 Free PMC article.
-
An animal model of differential genetic risk for methamphetamine intake.Front Neurosci. 2015 Sep 23;9:327. doi: 10.3389/fnins.2015.00327. eCollection 2015. Front Neurosci. 2015. PMID: 26441502 Free PMC article. Review.
-
Identification of Treatment Targets in a Genetic Mouse Model of Voluntary Methamphetamine Drinking.Int Rev Neurobiol. 2016;126:39-85. doi: 10.1016/bs.irn.2016.02.001. Epub 2016 Mar 7. Int Rev Neurobiol. 2016. PMID: 27055611 Review.
Cited by
-
Robust aversive effects of trace amine-associated receptor 1 activation in mice.Neuropsychopharmacology. 2023 Sep;48(10):1446-1454. doi: 10.1038/s41386-023-01578-4. Epub 2023 Apr 13. Neuropsychopharmacology. 2023. PMID: 37055488 Free PMC article.
-
Confirmation of a Causal Taar1 Allelic Variant in Addiction-Relevant Methamphetamine Behaviors.Front Psychiatry. 2021 Aug 26;12:725839. doi: 10.3389/fpsyt.2021.725839. eCollection 2021. Front Psychiatry. 2021. PMID: 34512422 Free PMC article.
-
Combined and sequential effects of alcohol and methamphetamine in animal models.Neurosci Biobehav Rev. 2021 Dec;131:248-269. doi: 10.1016/j.neubiorev.2021.09.019. Epub 2021 Sep 17. Neurosci Biobehav Rev. 2021. PMID: 34543650 Free PMC article. Review.
-
Dopamine D2 autoreceptor interactome: Targeting the receptor complex as a strategy for treatment of substance use disorder.Pharmacol Ther. 2020 Sep;213:107583. doi: 10.1016/j.pharmthera.2020.107583. Epub 2020 May 27. Pharmacol Ther. 2020. PMID: 32473160 Free PMC article. Review.
References
-
- Stenbacka M, Leifman A, Romelsjö A. (2010) Mortality and cause of death among 1705 illicit drug users: A 37 year follow up. Drug Alcohol Rev 29, 21–27. - PubMed
-
- Aoyama N, Takahashi N, Kitaichi K, et al. (2006) Association between gene polymorphisms of SLC22A3 and methamphetamine use disorder. Alcohol Clin Exp Res 30, 1644–1649. - PubMed
-
- Kim M, Custodio RJ, Botanas CJ, et al. (2018) The circadian gene, Per2, influences methamphetamine sensitization and reward through the dopaminergic system in the striatum of mice. Addict Biol 24, 946–957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

