A simple, versatile and robust centrifugation-based filtration protocol for the isolation and quantification of α-synuclein monomers, oligomers and fibrils: Towards improving experimental reproducibility in α-synuclein research
- PMID: 31925956
- PMCID: PMC7155127
- DOI: 10.1111/jnc.14955
A simple, versatile and robust centrifugation-based filtration protocol for the isolation and quantification of α-synuclein monomers, oligomers and fibrils: Towards improving experimental reproducibility in α-synuclein research
Abstract
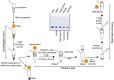
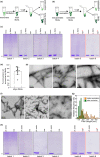
Increasing evidence suggests that the process of alpha-synuclein (α-syn) aggregation from monomers into amyloid fibrils and Lewy bodies, via oligomeric intermediates plays an essential role in the pathogenesis of different synucleinopathies, including Parkinson's disease (PD), multiple system atrophy and dementia with Lewy bodies (DLB). However, the nature of the toxic species and the mechanisms by which they contribute to neurotoxicity and disease progression remain elusive. Over the past two decades, significant efforts and resources have been invested in studies aimed at identifying and targeting toxic species along the pathway of α-syn fibrillization. Although this approach has helped to advance the field and provide insights into the biological properties and toxicity of different α-syn species, many of the fundamental questions regarding the role of α-syn aggregation in PD remain unanswered, and no therapeutic compounds targeting α-syn aggregates have passed clinical trials. Several factors have contributed to this slow progress, including the complexity of the aggregation pathways and the heterogeneity and dynamic nature of α-syn aggregates. In the majority of experiment, the α-syn samples used contain mixtures of α-syn species that exist in equilibrium and their ratio changes upon modifying experimental conditions. The failure to quantitatively account for the distribution of different α-syn species in different studies has contributed not only to experimental irreproducibility but also to misinterpretation of results and misdirection of valuable resources. Towards addressing these challenges and improving experimental reproducibility in Parkinson's research, we describe here a simple centrifugation-based filtration protocol for the isolation, quantification and assessment of the distribution of α-syn monomers, oligomers and fibrils, in heterogeneous α-syn samples of increasing complexity. The protocol is simple, does not require any special instrumentation and can be performed rapidly on multiple samples using small volumes. Here, we present and discuss several examples that illustrate the applications of this protocol and how it could contribute to improving the reproducibility of experiments aimed at elucidating the structural basis of α-syn aggregation, seeding activity, toxicity and pathology spreading. This protocol is applicable, with slight modifications, to other amyloid-forming proteins.
Keywords: Parkinson's disease; alpha-synuclein; amyloid fibrils; oligomers and monomers.
© 2020 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Figures



References
-
- Alam, P. , Bousset, L. , Melki, R. , & Otzen, D. E. (2019). Alpha‐synuclein oligomers and fibrils: A spectrum of species, a spectrum of toxicities. Journal of Neurochemistry, 150, 522–534. - PubMed
-
- Bieschke, J. , Russ, J. , Friedrich, R. P. , Ehrnhoefer, D. E. , Wobst, H. , Neugebauer, K. , & Wanker, E. E. (2010). EGCG remodels mature alpha‐synuclein and amyloid‐beta fibrils and reduces cellular toxicity. Proceedings of the National Academy of Sciences of the United States of America, 107, 7710–7715. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

