Neural connectivity between the hypothalamic supramammillary nucleus and appetite- and motivation-related regions of the rat brain
- PMID: 31925973
- PMCID: PMC7065010
- DOI: 10.1111/jne.12829
Neural connectivity between the hypothalamic supramammillary nucleus and appetite- and motivation-related regions of the rat brain
Abstract
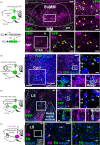
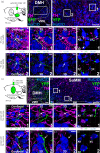
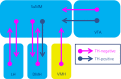
The supramammillary nucleus (SuM) has an emerging role in appetite control. We have shown that the rat SuM is activated during hunger or food anticipation, or by ghrelin administration. In the present study, we characterised the connectivity between the SuM and key appetite- and motivation-related nuclei in the rat. In adult wild-type rats, or rats expressing Cre recombinase under the control of the tyrosine hydroxylase (TH) promoter (TH-Cre rats), we used c-Fos immunohistochemistry to visualise and correlate the activation of medial SuM (SuMM) with activation in the lateral hypothalamic area (LH), the dorsomedial hypothalamus (DMH) or the ventral tegmental area (VTA) after voluntary consumption of a high-sugar, high-fat food. To determine neuroanatomical connectivity, we used retrograde and anterograde tracing methods to specifically investigate the neuronal inputs and outputs of the SuMM. After consumption of the food there were positive correlations between c-Fos expression in the SuMM and the LH, DMH and VTA (P = 0.0001, 0.01 and 0.004). Using Fluoro-Ruby as a retrograde tracer, we demonstrate the existence of inputs from the LH, DMH, VTA and ventromedial hypothalamus (VMH) to the SuMM. The SuMM showed reciprocal inputs to the LH and DMH, and we identified a TH-positive output from SuMM to DMH. We co-labelled retrogradely-labelled sections for TH in the VMH, or for TH, orexin and melanin-concentrating hormone in the LH and DMH. However, we did not observe any colocalisation of immunoreactivity with any retrogradely-labelled cells. Viral mapping in TH-Cre rats confirms the existence of a reciprocal SuMM-DMH connection and shows that TH-positive cells project from the SuMM and VTA to the lateral septal area and cingulate cortex, respectively. These data provide evidence for the connectivity of the SuMM to brain regions involved in appetite control, and form the foundation for functional and behavioural studies aiming to further characterise the brain circuitry controlling eating behaviours.
Keywords: food; motivation; neuroanatomy; supramammillary nucleus; tracing.
© 2020 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Figures



Similar articles
-
GLP-1 modulates the supramammillary nucleus-lateral hypothalamic neurocircuit to control ingestive and motivated behavior in a sex divergent manner.Mol Metab. 2019 Feb;20:178-193. doi: 10.1016/j.molmet.2018.11.005. Epub 2018 Nov 27. Mol Metab. 2019. PMID: 30528281 Free PMC article.
-
Peripheral injection of CCK-8S induces Fos expression in the dorsomedial hypothalamic nucleus in rats.Brain Res. 2006 Oct 30;1117(1):109-17. doi: 10.1016/j.brainres.2006.08.092. Epub 2006 Sep 26. Brain Res. 2006. PMID: 17005163
-
Activation of the rat hypothalamic supramammillary nucleus by food anticipation, food restriction or ghrelin administration.J Neuroendocrinol. 2019 Jul;31(7):e12676. doi: 10.1111/jne.12676. Epub 2019 Feb 11. J Neuroendocrinol. 2019. PMID: 30580497
-
FoxP2 brainstem neurons project to sodium appetite regulatory sites.J Chem Neuroanat. 2011 Sep;42(1):1-23. doi: 10.1016/j.jchemneu.2011.05.003. Epub 2011 May 13. J Chem Neuroanat. 2011. PMID: 21605659 Free PMC article. Review.
-
How orexin signals bias action: Hypothalamic and accumbal circuits.Brain Res. 2020 Mar 15;1731:145943. doi: 10.1016/j.brainres.2018.09.011. Epub 2018 Sep 8. Brain Res. 2020. PMID: 30205111 Review.
Cited by
-
The rostral intralaminar nuclear complex of the thalamus supports striatally mediated action reinforcement.Elife. 2023 Apr 6;12:e83627. doi: 10.7554/eLife.83627. Elife. 2023. PMID: 37022333 Free PMC article.
-
To eat or not to eat: A role for ghrelin and LEAP2 in eating disorders?Neurosci Appl. 2024 Feb 27;3:104045. doi: 10.1016/j.nsa.2024.104045. eCollection 2024. Neurosci Appl. 2024. PMID: 40656102 Free PMC article. Review.
-
Local circuit allowing hypothalamic control of hippocampal area CA2 activity and consequences for CA1.Elife. 2021 May 18;10:e63352. doi: 10.7554/eLife.63352. Elife. 2021. PMID: 34003113 Free PMC article.
-
Tyrosine Hydroxylase Knockdown at the Hypothalamic Supramammillary Nucleus Area Induces Obesity and Glucose Intolerance.Neuroendocrinology. 2024;114(5):483-510. doi: 10.1159/000535944. Epub 2023 Dec 21. Neuroendocrinology. 2024. PMID: 38128505 Free PMC article.
-
Assessing the treatment of cannabidiolic acid methyl ester: a stable synthetic analogue of cannabidiolic acid on c-Fos and NeuN expression in the hypothalamus of rats.J Cannabis Res. 2021 Jul 12;3(1):31. doi: 10.1186/s42238-021-00081-1. J Cannabis Res. 2021. PMID: 34253253 Free PMC article.
References
-
- Le May MV, Hume C, Sabatier N, et al. Activation of the rat hypothalamic supramammillary nucleus by food anticipation, food restriction or ghrelin administration. J Neuroendocrinol. 2018;31:e12676. - PubMed
-
- Vogel H, Wolf S, Rabasa C, et al. GLP‐1 and estrogen conjugate acts in the supramammillary nucleus to reduce food‐reward and body weight. Neuropharmacology. 2016;110:396‐406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

