On the epigenetic role of guanosine oxidation
- PMID: 31926624
- PMCID: PMC6926346
- DOI: 10.1016/j.redox.2019.101398
On the epigenetic role of guanosine oxidation
Abstract
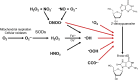
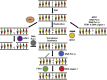
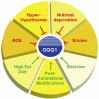
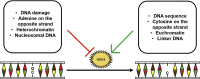
Chemical modifications of DNA and RNA regulate genome functions or trigger mutagenesis resulting in aging or cancer. Oxidations of macromolecules, including DNA, are common reactions in biological systems and often part of regulatory circuits rather than accidental events. DNA alterations are particularly relevant since the unique role of nuclear and mitochondrial genome is coding enduring and inheritable information. Therefore, an alteration in DNA may represent a relevant problem given its transmission to daughter cells. At the same time, the regulation of gene expression allows cells to continuously adapt to the environmental changes that occur throughout the life of the organism to ultimately maintain cellular homeostasis. Here we review the multiple ways that lead to DNA oxidation and the regulation of mechanisms activated by cells to repair this damage. Moreover, we present the recent evidence suggesting that DNA damage caused by physiological metabolism acts as epigenetic signal for regulation of gene expression. In particular, the predisposition of guanine to oxidation might reflect an adaptation to improve the genome plasticity to redox changes.
Keywords: Guanosine oxidation; Histone modifications; Oxidative stress; Transcription.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Steenken S., Jovanovic S.V. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997;119:617–618.
-
- Baik M.H., Silverman J.S., Yang I.V., Ropp P.A., Szalai V.A., Yang W., Thorp W.H.J. Using density functional theory to design DNA base analogues with low oxidation potentials. J. Phys. Chem. B. 2001;105(27):6437–6444.
-
- Hall D.B., Holmlin R.E., Barton J.K. Oxidative DNA damage through long-range electron transfer. Nature. 1996;382:731–735. - PubMed

