Neuronal vulnerability to fetal hypoxia-reoxygenation injury and motor deficit development relies on regional brain tetrahydrobiopterin levels
- PMID: 31926630
- PMCID: PMC6928344
- DOI: 10.1016/j.redox.2019.101407
Neuronal vulnerability to fetal hypoxia-reoxygenation injury and motor deficit development relies on regional brain tetrahydrobiopterin levels
Abstract
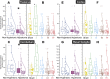
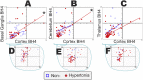
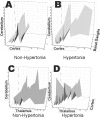
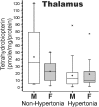
Hypertonia is pathognomonic of cerebral palsy (CP), often caused by brain injury before birth. To understand the early driving events of hypertonia, we utilized magnetic resonance imaging (MRI) assessment of early critical brain injury in rabbit fetuses (79% term) that will predict hypertonia after birth following antenatal hypoxia-ischemia. We examined if individual variations in the tetrahydrobiopterin cofactor in the parts of the brain controlling motor function could indicate a role in specific damage to motor regions and disruption of circuit integration as an underlying mechanism for acquiring motor disorders, which has not been considered before. The rabbit model mimicked acute placental insufficiency and used uterine ischemia at a premature gestation. MRI during the time of hypoxia-ischemia was used to differentiate which individual fetal brains would become hypertonic. Four brain regions collected immediately after hypoxia-ischemia or 48 h later were analyzed in a blinded fashion. Age-matched sham-operated animals were used as controls. Changes in the reactive nitrogen species and gene expression of the tetrahydrobiopterin biosynthetic enzymes in brain regions were also studied. We found that a combination of low tetrahydrobiopterin content in the cortex, basal ganglia, cerebellum, and thalamus brain regions, but not a unique low threshold of tetrahydrobiopterin, contributed etiologically to hypertonia. The biggest contribution was from the thalamus. Evidence for increased reactive nitrogen species was found in the cortex. By 48 h, tetrahydrobiopterin and gene expression levels in the different parts of the brain were not different between MRI stratified hypertonia and non-hypertonia groups. Sepiapterin treatment given to pregnant dams immediately after hypoxia-ischemia ameliorated hypertonia and death. We conclude that a developmental tetrahydrobiopterin variation is necessary with fetal hypoxia-ischemia and is critical for disrupting normal motor circuits that develop into hypertonia. The possible mechanistic pathway involves reactive nitrogen species.
Keywords: Cerebral palsy; Fetal brain; Free radicals; Hypertonia; Infant newborn; Sepiapterin.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None other than support by grants from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA under grant numbers R01 NS081936 (Vasquez-Vivar, Tan) and R56 NS100088 (Tan).
Figures






References
-
- Drobyshevsky A., Derrick M., Prasad P.V., Ji X., Englof I., Tan S. Fetal brain magnetic resonance imaging response acutely to hypoxia-ischemia predicts postnatal outcome. Ann. Neurol. 2007;61:307–314. - PubMed
-
- Elzaouk L., Leimbacher W., Turri M., Ledermann B., Burki K., Blau N., Thony B. Dwarfism and low insulin-like growth factor-1 due to dopamine depletion in Pts-/- mice rescued by feeding neurotransmitter precursors and H4-biopterin. J. Biol. Chem. 2003;278:28303–28311. - PubMed
-
- Shintaku H. Disorders of tetrahydrobiopterin metabolism and their treatment. Curr. Drug Metabol. 2002;3:123–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

