7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo
- PMID: 31926632
- PMCID: PMC6928325
- DOI: 10.1016/j.redox.2019.101378
7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo
Abstract
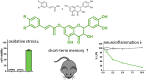
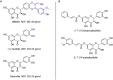
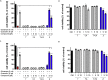
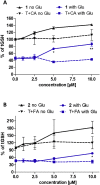
Alzheimer's disease (AD) is a multifactorial disease and the most common form of dementia. There are no treatments to cure, prevent or slow down the progression of the disease. Natural products hold considerable interest for the development of preventive neuroprotectants to treat neurodegenerative disorders like AD, due to their low toxicity and general beneficial effects on human health with their anti-inflammatory and antioxidant features. In this work we describe regioselective synthesis of 7-O-ester hybrids of the flavonoid taxifolin with the phenolic acids cinnamic and ferulic acid, namely 7-O-cinnamoyltaxifolin and 7-O-feruloyltaxifolin. The compounds show pronounced overadditive neuroprotective effects against oxytosis, ferroptosis and ATP depletion in the murine hippocampal neuron HT22 cell model. Furthermore, 7-O-cinnamoyltaxifolin and 7-O-feruloyltaxifolin reduced LPS-induced neuroinflammation in BV-2 microglia cells as assessed by effects on the levels of NO, IL6 and TNFα. In all in vitro assays the 7-O-esters of taxifolin and ferulic or cinnamic acid showed strong overadditive activity, significantly exceeding the effects of the individual components and the equimolar mixtures thereof, which were almost inactive in all of the assays at the tested concentrations. In vivo studies confirmed this overadditive effect. Treatment of an AD mouse model based on the injection of oligomerized Aβ25-35 peptide into the brain to cause neurotoxicity and subsequently memory deficits with 7-O-cinnamoyltaxifolin or 7-O-feruloyltaxifolin resulted in improved performance in an assay for short-term memory as compared to vehicle and mice treated with the respective equimolar mixtures. These results highlight the benefits of natural product hybrids as a novel compound class with potential use for drug discovery in neurodegenerative diseases due to their pharmacological profile that is distinct from the individual natural components.
Keywords: Alzheimer's disease; Flavonoids; In vivo studies; Microglia; Natural product hybrids; Phenolic acids.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





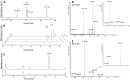
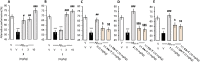

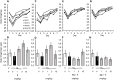
References
-
- World Alzheimer report 2018; Alzheimer's disease international. https://www.alz.co.uk/research/world-report-2018
-
- Doig A.J., Del Castillo-Frias M.P., Berthoumieu O., Tarus B., Nasica-Labouze J., Sterpone F., Nguyen P.H., Hooper N.M., Faller P., Derreumaux P. Why is research on amyloid-beta failing to give new drugs for Alzheimer’s disease? ACS Chem. Neurosci. 2017;8(7):1435–1437. - PubMed
-
- Agostinho P., Cunha R.A., Oliveira C.N. Oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharmaceut. Des. 2010;16(25):2766–2778. - PubMed
-
- Currais A., Chiruta C., Goujon-Svrzic M., Costa G., Santos T., Batista M.T., Paiva J., do Ceu Madureira M., Maher P. Screening and identification of neuroprotective compounds relevant to alzheimers disease from medicinal plants of S. Tomé e Príncipe. J. Ethnopharmacol. 2014;155(1):830–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

