Tumor-associated O-glycans of MUC1: Carriers of the glyco-code and targets for cancer vaccine design
- PMID: 31926647
- PMCID: PMC7160022
- DOI: 10.1016/j.smim.2020.101389
Tumor-associated O-glycans of MUC1: Carriers of the glyco-code and targets for cancer vaccine design
Abstract
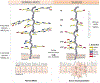
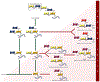
The transformation from normal to malignant phenotype in human cancers is associated with aberrant cell-surface glycosylation. It has frequently been reported that MUC1, the heavily glycosylated cell-surface mucin, is altered in both, expression and glycosylation pattern, in human carcinomas of the epithelium. The presence of incomplete or truncated glycan structures, often capped by sialic acid, commonly known as tumor-associated carbohydrate antigens (TACAs), play a key role in tumor initiation, progression, and metastasis. Accumulating evidence suggests that expression of TACAs is associated with tumor escape from immune defenses. In this report, we will give an overview of the oncogenic functions of MUC1 that are exerted through TACA interactions with endogenous carbohydrate-binding proteins (lectins). These interactions often lead to creation of a pro-tumor microenvironment, favoring tumor progression and metastasis, and tumor evasion. In addition, we will describe current efforts in the design of cancer vaccines with special emphasis on synthetic MUC1 glycopeptide vaccines. Analysis of the key factors that govern structure-based design of immunogenic MUC1 glycopeptide epitopes are described. The role of TACA type, position, and density on observed humoral and cellular immune responses is evaluated.
Keywords: Immune response; Lectins; MUC1; Tumor-associated carbohydrate antigens; Vaccines.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing financial interests.
Figures


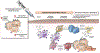

References
-
- Hattrup CL, Gendler SJ, Structure and function of the cell surface (tethered) mucins, Annu. Rev. Physiol. 70 (2008) 431–457. - PubMed
-
- Gendler SJ, Spicer A, Epithelial mucin genes, Annu. Rev. Physiol. 57 (1995) 607–634. - PubMed
-
- Gendler S, MUC1, The renaissance molecule, J. Mammary Gland Biol. 6(3) (2001) 339–353. - PubMed
-
- Hanisch F, Muller S, MUC1: the polymorphic appearance of a human mucin, Glycobiology 10(5) (2000) 439–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

