Deep brain stimulation in treatment resistant schizophrenia: A pilot randomized cross-over clinical trial
- PMID: 31927311
- PMCID: PMC6953640
- DOI: 10.1016/j.ebiom.2019.11.029
Deep brain stimulation in treatment resistant schizophrenia: A pilot randomized cross-over clinical trial
Abstract
Background: Up to 30% of patients with schizophrenia are resistant to antipsychotic drug treatment, with 60% of such cases also failing to respond to clozapine. Deep brain stimulation (DBS) has been used in treatment resistant patients with other psychiatric disorders, but there is a lack of trials in schizophrenia, partly due to uncertainties over where to site the electrodes. This trial aimed to examine the effectiveness of nucleus accumbens (NAcc) and subgenual anterior cingulate cortex (subgenual ACC) targeted DBS; the primary outcome measure was PANSS total score, as assessed fortnightly.
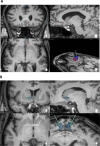
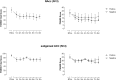
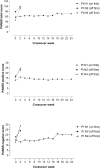
Methods: Eight patients with schizophrenia, who met criteria for treatment resistance and were also resistant to/intolerant of clozapine, were randomly assigned using central allocation to receive DBS in the NAcc or subgenual ACC. An open stabilization phase lasting at least six months was followed by a randomized double-blind crossover phase lasting 24 weeks in those who met symptomatic improvement criteria. The primary end-point was a 25% improvement in PANSS total score. (ClinicalTrials.gov Identifier: NCT02377505; trial completed).
Findings: One implanted patient did not receive DBS due to complications of surgery. Of the remaining 7 patients, 2/3 with NAcc and 2/4 with subgenual ACC electrode placements met the symptomatic improvement criteria (58% and 86%, and 37% and 68% improvement in PANSS total score, respectively). Three of these patients entered the crossover phase and all showed worsening when the stimulation was discontinued. The fourth patient worsened after the current was switched off accidentally without her or the investigators' knowledge. Physical adverse events were uncommon, but two patients developed persistent psychiatric adverse effects (negative symptoms/apathy and mood instability, respectively).
Interpretation: These preliminary findings point to the possibility of DBS having therapeutic effects in patients with schizophrenia who do not respond to any other treatment. Larger trials with careful attention to blinding will be necessary to establish the extent of the benefits and whether these can be achieved without psychiatric side-effects.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Kahn R.S., Sommer I.E., Murray R.M. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. - PubMed
-
- Elkis H. Treatment-resistant schizophrenia. Psychiat Clin N Am. 2007;30:511–533. - PubMed
-
- Buchanan R.W., Javitt D.C., Marder S.R. The cognitive and negative symptoms in schizophrenia trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. - PubMed
-
- Kinon B.J., Zhang L., Millen B.A. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with dsm-iv schizophrenia. J Clin Psychopharmacol. 2011;31:349–355. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

