Men's Sexual Health Questionnaire score changes vs spontaneous sexual adverse event reporting in men treated with dutasteride/tamsulosin combination therapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A post hoc analysis of a prospective, randomised, placebo-controlled study
- PMID: 31927774
- PMCID: PMC7187250
- DOI: 10.1111/ijcp.13480
Men's Sexual Health Questionnaire score changes vs spontaneous sexual adverse event reporting in men treated with dutasteride/tamsulosin combination therapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A post hoc analysis of a prospective, randomised, placebo-controlled study
Abstract
Aim: To assess the impact of baseline characteristics on Men's Sexual Health Questionnaire (MSHQ) total scores and to evaluate the clinical relevance of MSHQ changes and their association with spontaneously reported sexual adverse events (SexAEs) in patients with benign prostatic hyperplasia.
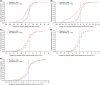
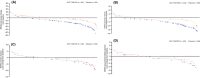
Methods: This was a post hoc analysis of the Phase 4 FDC116115 study, in which patients aged ≥50 years were randomised 1:1 to receive a fixed-dose combination of dutasteride 0.5 mg and tamsulosin 0.4 mg (DUT-TAM FDC), or placebo. End-points included: change in MSHQ total scores by baseline characteristics and SexAEs; cumulative distribution function for change from baseline to month 12 in MSHQ total score and the ejaculation, erection, satisfaction and sexual desire (libido) domain scores; and relationship between changes in MSHQ scores and SexAEs.
Results: The intent-to-treat population comprised 489 patients (DUT-TAM FDC, n = 243; placebo, n = 246). The mean reduction in total MSHQ score was greater in patients with SexAEs across both groups, compared with patients without SexAEs. Most patients reporting any SexAE (86% DUT-TAM FDC, 67% placebo) had a worsening of the MSHQ total score at month 12 compared with baseline. Specifically, 90% (DUT-TAM FDC) and 75% (placebo) of patients reporting an ejaculation SexAE and 73% (DUT-TAM FDC) and 87% (placebo) of patients reporting an erection SexAE had a worsening of MSHQ ejaculation and erection domain scores, respectively, at month 12. A threshold effect for incident SexAE was observed; patients showing a decrease of approximately 6-10 points in the total MSHQ score were more likely to report SexAEs.
Conclusion: Findings support the clinical utility of the MSHQ tool in assessing the impact of DUT-TAM on sexual function by linking numerical changes in MSHQ scores to spontaneously reported SexAEs for the first time. The threshold effect for incidence of SexAEs warrants further investigation to determine its clinical relevance.
© 2020 The Authors. International Journal of Clinical Practice published by John Wiley & Sons Ltd.
Conflict of interest statement
CGR is a consultant for GSK, Lilly, Procept, NxThera, Neotract and Sophiris and has previously received grants or research support from NxThera, Neotract, Procept and Astellas. RCR received research support from Bayer Healthcare, Eli Lilly, Shionogi and Pfizer. MJM is a current employee of GSK and owns stock/shares in GSK. JMP‐M is a current employee of GSK and owns stocks/shares in GSK. THW is an employee of PAREXEL International (Durham, NC, USA) and owns stocks/shares in GSK. ZL is a current employee of GSK and owns stocks/shares in GSK. FG is a consultant for Pfizer, Sanofi, Menarini, Recordati and Ipsen.
Figures

Comment in
-
Benign Prostatic Hyperplasia.J Urol. 2021 May;205(5):1490-1492. doi: 10.1097/JU.0000000000001665. Epub 2021 Feb 24. J Urol. 2021. PMID: 33625910 No abstract available.
References
-
- Gravas S, Bach T, Bachmann A, et al. EAU guidelines on Management of Non‐Neurogenic Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO) 2019 [cited 2019 October]. Retrieved from https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-the-Management-o...
-
- Kirby RS, Roehrborn C, Boyle P, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003;61(1):119‐126. 10.1016/S0090-4295(02)02114-3 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
