An Improved Methodology to Evaluate Cell and Molecular Signals in the Reparative Callus During Fracture Healing
- PMID: 31928129
- PMCID: PMC7045301
- DOI: 10.1369/0022155419900915
An Improved Methodology to Evaluate Cell and Molecular Signals in the Reparative Callus During Fracture Healing
Abstract
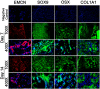
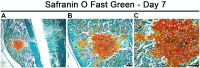
Approximately 5% to 10% of all bone fractures do not heal completely, contributing to significant patient suffering and medical costs. Even in healthy individuals, fracture healing is associated with significant downtime and loss of productivity. However, no pharmacological treatments are currently available to promote efficient bone healing. A better understanding of the underlying molecular mechanisms is crucial for developing novel therapies to hasten healing. The early reparative callus that forms around the site of bone injury is a fragile tissue consisting of shifting cell populations held together by loose connective tissue. The delicate callus is challenging to section and is vulnerable to disintegration during the harsh steps of immunostaining, namely, decalcification, deparaffinization, and antigen retrieval. Here, we describe an improved methodology for processing early-stage fracture calluses and immunofluorescence labeling of the sections to visualize the temporal (timing) and spatial (location) patterns of cellular and molecular events that regulate bone healing. This method has a short turnaround time from sample collection to microscopy as it does not require lengthy decalcification. It preserves the structural integrity of the fragile callus as the method does not entail deparaffinization or harsh methods of antigen retrieval. Our method can be adapted for high-throughput screening of drugs that promote efficacious bone healing.
Keywords: bone matrix; cartilage; chondrocytes; cryosection; fluorescence microscopy; fracture callus; immunofluorescence labeling; osteoblasts; safranin O/fast green.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

