Hydrogen sulfide mitigates myocardial inflammation by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation in diabetic rats
- PMID: 31928360
- PMCID: PMC7045329
- DOI: 10.1177/1535370219899899
Hydrogen sulfide mitigates myocardial inflammation by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation in diabetic rats
Abstract
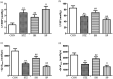
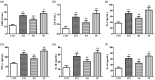
Inflammation plays a crucial part in hyperglycemia-induced myocardial damage. Hydrogen sulfide has been found to possess multiple biological activities in previous studies. This study investigated whether hydrogen sulfide conferred cardiac protection against damage in a diabetic rat model by inhibiting nucleotide-binding oligomerization domain-like receptor protein (NLRP) 3 inflammasome activation. Male animals were assigned to control, streptozotocin, streptozotocin + sodium hydrosulfide, and streptozotocin + DL-propargylglycine groups. Animals in the three streptozotocin groups were administrated 55 mg/kg streptozotocin by intraperitoneal injection. Streptozotocin + sodium hydrosulfide and streptozotocin + propargylglycine groups were treated with sodium hydrosulfide (56 μmol/kg) and propargylglycine (40 mg/kg), respectively, for four weeks. Estimation of fasting blood glucose, heart-weight/body-weight, cardiac function, and histopathological analysis, and measurement of myocardial enzymes were done to evaluate the degree of cardiac injury. In order to investigate the redox changes, the levels of total antioxidant capacity, malondialdehyde and lipid peroxidation, and the activities of superoxide dismutase, catalase, and glutathione peroxidase were assessed; the protein expression levels of Thioredoxin and Thioredoxin-interacting protein were measured in myocardial tissue. In addition, inflammatory reactions were assessed by measuring the concentration levels of interleukin-6, tumor necrosis factor-α, interleukin-1β, and interleukin-18 in serum and the expression levels of NLRP3 inflammasome complex-associated proteins in cardiac tissue. In the heart, hyperglycemia significantly induced cardiac dysfunction and injury, redox perturbation, and aggravation of inflammatory reactions. However, except for fasting blood glucose, treatment with sodium hydrosulfide significantly ameliorated these alterations, whereas treatment with propargylglycine further aggravated these alterations. This study highlights the protective properties of hydrogen sulfide against hyperglycemia-induced cardiac injury, and its possible mechanism was shown to involve negative regulation of Thioredoxin-interacting protein-mediated NLRP3 inflammasome activation.
Impact statement: Diabetic cardiomyopathy is a serious complication of diabetic patients, accompanied by chronic inflammation. The nucleotide-binding oligomerization domain-like receptor protein (NLRP) 3 inflammasome complex is involved in the progression of the inflammatory response of diabetes, including diabetic cardiomyopathy. Hydrogen sulfide (H2S) is a novel endogenous gas messenger. Several pieces of evidence have exhibited that H2S exerts anti-oxidant and anti-inflammatory activities against hyperglycemia-induced myocardial injury, but the mechanism remains unclear. The current study indicated that H2S protected the myocardium against hyperglycemia-induced injury by preventing Thioredoxin-interacting protein (TXNIP)-mediated NLRP3 inflammasome complex activation. The inhibition of TXNIP-mediated NLRP3 inflammasome complex would be an efficient therapy for H2S treatment in diabetic cardiomyocytes.
Keywords: Hydrogen sulfide; diabetes mellitus; inflammation; nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome; oxidative stress; rat.
Figures






Similar articles
-
Protective effect of exogenous hydrogen sulfide on diaphragm muscle fibrosis in streptozotocin-induced diabetic rats.Exp Biol Med (Maywood). 2020 Aug;245(14):1280-1289. doi: 10.1177/1535370220931038. Epub 2020 Jun 3. Exp Biol Med (Maywood). 2020. PMID: 32493122 Free PMC article.
-
Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation.J Cell Mol Med. 2018 Sep;22(9):4437-4448. doi: 10.1111/jcmm.13743. Epub 2018 Jul 11. J Cell Mol Med. 2018. PMID: 29993180 Free PMC article.
-
Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model.Cardiovasc Drugs Ther. 2014 Feb;28(1):33-43. doi: 10.1007/s10557-013-6498-1. Cardiovasc Drugs Ther. 2014. PMID: 24254031
-
[Advances in mechanisms for NLRP3 inflammasomes regulation].Yao Xue Xue Bao. 2016 Oct;51(10):1505-12. Yao Xue Xue Bao. 2016. PMID: 29924571 Review. Chinese.
-
Biochemical regulation of the inflammasome.Crit Rev Biochem Mol Biol. 2012 Sep;47(5):424-43. doi: 10.3109/10409238.2012.694844. Epub 2012 Jun 11. Crit Rev Biochem Mol Biol. 2012. PMID: 22681257 Review.
Cited by
-
Inflammasomes: novel therapeutic targets for metabolic syndrome?Front Endocrinol (Lausanne). 2025 May 13;16:1569579. doi: 10.3389/fendo.2025.1569579. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40433411 Free PMC article. Review.
-
Inulin Reduces Kidney Damage in Type 2 Diabetic Mice by Decreasing Inflammation and Serum Metabolomics.J Diabetes Res. 2024 May 2;2024:1222395. doi: 10.1155/2024/1222395. eCollection 2024. J Diabetes Res. 2024. PMID: 38725443 Free PMC article.
-
The Role of the Signaling Pathways Involved in the Protective Effect of Exogenous Hydrogen Sulfide on Myocardial Ischemia-Reperfusion Injury.Front Cell Dev Biol. 2021 Aug 30;9:723569. doi: 10.3389/fcell.2021.723569. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34527675 Free PMC article. Review.
-
Epigallocatechin-3-gallate ameliorates renal endoplasmic reticulum stress-mediated inflammation in type 2 diabetic rats.Exp Biol Med (Maywood). 2022 Aug;247(16):1410-1419. doi: 10.1177/15353702221106479. Epub 2022 Jul 1. Exp Biol Med (Maywood). 2022. PMID: 35775606 Free PMC article.
-
Recent Advances in Molecular Research on Hydrogen Sulfide (H2S) Role in Diabetes Mellitus (DM)-A Systematic Review.Int J Mol Sci. 2022 Jun 16;23(12):6720. doi: 10.3390/ijms23126720. Int J Mol Sci. 2022. PMID: 35743160 Free PMC article.
References
-
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013; 93:137–88 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

