LOXL1 folding in exfoliation glaucoma
- PMID: 31928728
- PMCID: PMC7589528
- DOI: 10.1016/bs.apcsb.2019.09.005
LOXL1 folding in exfoliation glaucoma
Abstract
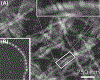
Exfoliation syndrome (XFS) is an age-related disease defined by the deposition of aggregated fibrous material (XFM) in the peri-cellular space. Principal morbidity occurs in the eye, where XFM accumulates on the anterior ocular tissues. GWAS have found that certain genetic variants of lysyl oxidase-like 1 (LOXL1), a matrix cross-linking enzyme that is required for elastic fiber formation confer risk for the development of XFS, but are not a single causative factor as many genetically affected individuals do not develop XFS or subsequent glaucoma (XFG). We have found that XFG cells display defects in lysosomes, microtubules, autophagy, and mitochondria resembling defects found in cells from age-related syndromes, such as the main neurodegenerative diseases. In the majority of these diseases, the determining cellular factor is a protein containing intrinsically disordered regions (IDRs) and displaying a high propensity for aggregation. We have found that in XFG patient-derived cells, LOXL1 protein is actively subjected to autophagic clearance, suggesting that LOXL1 is undergoing aggregation. In silico analysis demonstrates that LOXL1's first 369 aa constitute an IDR with the highest disorder probability peak centering around the known risk positions. Experimentally, we have found over-expression of either unmodified LOXL1 or fluorescent chimeras preserving the well-structured N-terminus cause copious intracellular aggregation and that aggregation wanes when the high IDR peaks are deleted. Overall, our work suggests that XFS/G results from the aggregation of the LOXL1 protein coupled with a reduction of cellular proteostasis capabilities in aging, resulting in a chronic build-up of LOXL1-containing protein aggregates.
Keywords: Aggregopathy; Autophagy; Glaucoma; Intrinsically disordered regions; LOXL1.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
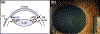




References
-
- Abu-Amero KK, Osman EA, Dewedar AS, Schmidt S, Allingham RR, & Al-Obeidan SA (2010). Analysis of LOXL1 polymorphisms in a Saudi Arabian population with pseudoexfoliation glaucoma. Molecular Vision, 16, 2805–2810. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21197115. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

