Structural Basis of Teneurin-Latrophilin Interaction in Repulsive Guidance of Migrating Neurons
- PMID: 31928845
- PMCID: PMC6978801
- DOI: 10.1016/j.cell.2019.12.014
Structural Basis of Teneurin-Latrophilin Interaction in Repulsive Guidance of Migrating Neurons
Abstract
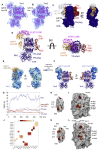
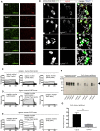
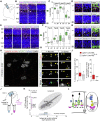
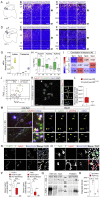
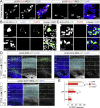
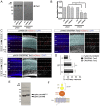
Teneurins are ancient metazoan cell adhesion receptors that control brain development and neuronal wiring in higher animals. The extracellular C terminus binds the adhesion GPCR Latrophilin, forming a trans-cellular complex with synaptogenic functions. However, Teneurins, Latrophilins, and FLRT proteins are also expressed during murine cortical cell migration at earlier developmental stages. Here, we present crystal structures of Teneurin-Latrophilin complexes that reveal how the lectin and olfactomedin domains of Latrophilin bind across a spiraling beta-barrel domain of Teneurin, the YD shell. We couple structure-based protein engineering to biophysical analysis, cell migration assays, and in utero electroporation experiments to probe the importance of the interaction in cortical neuron migration. We show that binding of Latrophilins to Teneurins and FLRTs directs the migration of neurons using a contact repulsion-dependent mechanism. The effect is observed with cell bodies and small neurites rather than their processes. The results exemplify how a structure-encoded synaptogenic protein complex is also used for repulsive cell guidance.
Keywords: FLRT; Latrophilin; Teneurin; adhesion; cortex development; neuronal migration; pyramidal neuron; radial glia; repulsion.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures









References
-
- Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25.
-
- Aldahmesh M.A., Mohammed J.Y., Al-Hazzaa S., Alkuraya F.S. Homozygous null mutation in ODZ3 causes microphthalmia in humans. Genet. Med. 2012;14:900–904. - PubMed
-
- Alkelai A., Olender T., Haffner-Krausz R., Tsoory M.M., Boyko V., Tatarskyy P., Gross-Isseroff R., Milgrom R., Shushan S., Blau I. A role for TENM1 mutations in congenital general anosmia. Clin. Genet. 2016;90:211–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

