Circuit Mechanisms Underlying Chromatic Encoding in Drosophila Photoreceptors
- PMID: 31928878
- PMCID: PMC6981066
- DOI: 10.1016/j.cub.2019.11.075
Circuit Mechanisms Underlying Chromatic Encoding in Drosophila Photoreceptors
Abstract
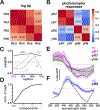
Spectral information is commonly processed in the brain through generation of antagonistic responses to different wavelengths. In many species, these color opponent signals arise as early as photoreceptor terminals. Here, we measure the spectral tuning of photoreceptors in Drosophila. In addition to a previously described pathway comparing wavelengths at each point in space, we find a horizontal-cell-mediated pathway similar to that found in mammals. This pathway enables additional spectral comparisons through lateral inhibition, expanding the range of chromatic encoding in the fly. Together, these two pathways enable efficient decorrelation and dimensionality reduction of photoreceptor signals while retaining maximal chromatic information. A biologically constrained model accounts for our findings and predicts a spatio-chromatic receptive field for fly photoreceptor outputs, with a color opponent center and broadband surround. This dual mechanism combines motifs of both an insect-specific visual circuit and an evolutionarily convergent circuit architecture, endowing flies with the ability to extract chromatic information at distinct spatial resolutions.
Keywords: Drosophila melanogaster; color model; color opponency; color vision; convergent evolution; horizontal cell; medulla; neural circuit; photon capture; photoreceptor.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors do not declare any conflicts of interest.
Figures
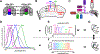




Comment in
-
Colour Vision: Self-Centered Fly Photoreceptors Communicate over Distances.Curr Biol. 2020 Jan 20;30(2):R78-R81. doi: 10.1016/j.cub.2019.11.050. Curr Biol. 2020. PMID: 31962082
References
-
- Buchsbaum G, Gottschalk A, Trichromacy, opponent colours coding and optimum colour information transmission in the retina, Proc R Soc L. B Biol Sci 220 (1983) 89–113. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

