In vitro endothelial hyperpermeability occurs early following traumatic hemorrhagic shock
- PMID: 31929146
- PMCID: PMC7504990
- DOI: 10.3233/CH-190642
In vitro endothelial hyperpermeability occurs early following traumatic hemorrhagic shock
Abstract
Background: Endothelial hyperpermeability is suggested to play a role in the development of microcirculatory perfusion disturbances and organ failure following hemorrhagic shock, but evidence is limited.
Objective: To study the effect of plasma from traumatic hemorrhagic shock patients on in vitro endothelial barrier function.
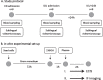
Methods: Plasma from traumatic hemorrhagic shock patients was obtained at the emergency department (ED), the intensive care unit (ICU), 24 h after ICU admission and from controls (n = 8). Sublingual microcirculatory perfusion was measured using incident dark field videomicroscopy at matching time points. Using electric cell-substrate impedance sensing, the effects of plasma exposure on in vitro endothelial barrier function of human endothelial cells were assessed.
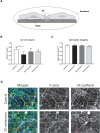
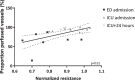
Results: Plasma from traumatic hemorrhagic shock patients collected at ED admission induced a 19% loss of in vitro endothelial resistance compared to plasma from controls (p < 0.001). This loss was due to reduced cell-cell contacts (p < 0.01). Plasma withdrawn at later time points did not affect endothelial barrier function (p > 0.99). Interestingly, in vitro endothelial resistance showed a positive association with in vivo microcirculatory perfusion (r = 0.56, p < 0.01).
Conclusions: Plasma from traumatic hemorrhagic shock patients obtained following ED admission, but not at later stages, induced in vitro endothelial hyperpermeability. This coincided with in vivo microcirculatory perfusion disturbances.
Keywords: Hemorrhagic shock; endothelial barrier function; endothelial permeability; microcirculation; plasma.
Figures

References
-
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. - PubMed
-
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–34. - PubMed
-
- Tachon G, Harrois A, Tanaka S, Kato H, Huet O, Pottecher J, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med. 2014;42(6):1433–41. - PubMed
-
- Hutchings SD, Naumann DN, Hopkins P, Mellis C, Riozzi O, Sartini S, et al. Microcirculatory impairment is associated with multiple organ dysfunction following traumatic haemorrhagic shock: The MICROSHOCK study. Crit Care Med. 2018;46(9):e889–96. - PubMed
-
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

