β-Lactolin, a Whey-Derived Lacto-Tetrapeptide, Prevents Alzheimer's Disease Pathologies and Cognitive Decline
- PMID: 31929163
- PMCID: PMC7081097
- DOI: 10.3233/JAD-190997
β-Lactolin, a Whey-Derived Lacto-Tetrapeptide, Prevents Alzheimer's Disease Pathologies and Cognitive Decline
Abstract
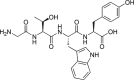
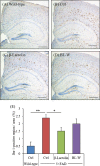
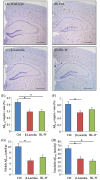
The prevention of age-related memory decline and dementia has been becoming a high priority because of the rapid growth in aging populations. Accumulating epidemiological and clinical studies indicate that intake of fermented dairy products rich in β-lactolin improves memory retrieval and executive function and attenuates cognitive decline in the elderly. However, the effects of long-term consumption of β-lactolin on Alzheimer's disease (AD) pathologies have not been investigated. In the present study, we examined the effects of β-lactolin and whey digestion rich in β-lactolin on AD pathology in 5×FAD transgenic mice and PS19 tauopathy mice. Intake of β-lactolin and whey digestion rich in β-lactolin reduced the levels of inflammatory cytokines, suppressed the infiltration of activated microglia, decreased the levels of amyloid-β, ameliorated impaired long-term object memory, and attenuated decreased synaptophysin, dopamine, brain-derived neurotrophic factor, and insulin-like growth factor 1 levels in the cortex in 5×FAD transgenic mice. In addition, intake of β-lactolin and whey digestion rich in β-lactolin improved behavioral abnormality and reduced the ratio of phosphorylated tau to total tau in the cortex in PS19 tauopathy mice. These findings indicate that consumption with β-lactolin and whey digestion rich in β-lactolin suppresses inflammation and attenuates AD pathology and cognitive impairment.
Keywords: Alzheimer’s disease; amyloid-β; cognitive function; inflammation; memory; microglia; peptide; tauopathy; β-lactolin.
Conflict of interest statement
Authors’ disclosures available online (
Figures



References
-
- Takashima A (2009) Amyloid-beta, tau, and dementia. J Alzheimers Dis 17, 729–736. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

