Efavirenz-Based Antiretroviral Therapy Reduces Artemether-Lumefantrine Exposure for Malaria Treatment in HIV-Infected Pregnant Women
- PMID: 31929402
- PMCID: PMC7061940
- DOI: 10.1097/QAI.0000000000002237
Efavirenz-Based Antiretroviral Therapy Reduces Artemether-Lumefantrine Exposure for Malaria Treatment in HIV-Infected Pregnant Women
Abstract
Background: The choice of malaria treatment for HIV-infected pregnant women receiving efavirenz-based antiretroviral therapy must consider the potential impact of drug interactions on antimalarial exposure and clinical response. The aim of this study was to investigate the effects of efavirenz on artemether-lumefantrine (AL) because no studies have isolated the impact of efavirenz for HIV-infected pregnant women.
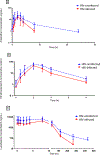
Methods: A prospective clinical pharmacokinetic (PK) study compared HIV-infected, efavirenz-treated pregnant women with HIV-uninfected pregnant women in Tororo, Uganda. All women received the standard 6-dose AL treatment regimen for Plasmodium falciparum malaria with intensive PK samples collected over 21 days and 42-days of clinical follow-up. PK exposure parameters were calculated for artemether, its active metabolite dihydroartemisinin (DHA), and lumefantrine to determine the impact of efavirenz.
Results: Nine HIV-infected and 30 HIV-uninfected pregnant women completed intensive PK evaluations. Relative to controls, concomitant efavirenz therapy lowered the 8-hour artemether concentration by 76% (P = 0.013), DHA peak concentration by 46% (P = 0.033), and day 7 and 14 lumefantrine concentration by 61% and 81% (P = 0.046 and 0.023), respectively. In addition, there were nonsignificant reductions in DHA area under the concentration-time curve0-8hr (35%, P = 0.057) and lumefantrine area under the concentration-time curve0-∞ (34%, P = 0.063) with efavirenz therapy.
Conclusions: Pregnant HIV-infected women receiving efavirenz-based antiretroviral therapy during malaria treatment with AL showed reduced exposure to both the artemisinin and lumefantrine. These data suggest that malaria and HIV coinfected pregnant women may require adjustments in AL dosage or treatment duration to achieve exposure comparable with HIV-uninfected pregnant women.
Conflict of interest statement
Figures
Similar articles
-
Efficacy of artemisinin-based combination therapy (ACT) in people living with HIV (PLHIV) diagnosed with uncomplicated Plasmodium falciparum malaria in Africa: a WWARN systematic review.Malar J. 2025 May 16;24(1):153. doi: 10.1186/s12936-025-05393-8. Malar J. 2025. PMID: 40380136 Free PMC article.
-
Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults.J Antimicrob Chemother. 2012 Sep;67(9):2213-21. doi: 10.1093/jac/dks207. Epub 2012 Jun 11. J Antimicrob Chemother. 2012. PMID: 22687893 Free PMC article. Clinical Trial.
-
Extended Treatment Duration of Artemether-Lumefantrine in Ugandan Children with HIV on Efavirenz-Based Antiretroviral Therapy: A Randomized Controlled Pharmacokinetic and Pharmacodynamic Trial.J Clin Pharmacol. 2025 Jul;65(7):909-922. doi: 10.1002/jcph.6193. Epub 2025 Jan 24. J Clin Pharmacol. 2025. PMID: 39853752 Clinical Trial.
-
Antiretroviral Choice for HIV Impacts Antimalarial Exposure and Treatment Outcomes in Ugandan Children.Clin Infect Dis. 2016 Aug 1;63(3):414-22. doi: 10.1093/cid/ciw291. Epub 2016 May 3. Clin Infect Dis. 2016. PMID: 27143666 Free PMC article.
-
Understanding the pharmacokinetics of Coartem.Malar J. 2009 Oct 12;8 Suppl 1(Suppl 1):S4. doi: 10.1186/1475-2875-8-S1-S4. Malar J. 2009. PMID: 19818171 Free PMC article. Review.
Cited by
-
Efficacy of artemisinin-based combination therapy (ACT) in people living with HIV (PLHIV) diagnosed with uncomplicated Plasmodium falciparum malaria in Africa: a WWARN systematic review.Malar J. 2025 May 16;24(1):153. doi: 10.1186/s12936-025-05393-8. Malar J. 2025. PMID: 40380136 Free PMC article.
-
Anti-malarial drugs: Mechanisms underlying their proarrhythmic effects.Br J Pharmacol. 2022 Dec;179(24):5237-5258. doi: 10.1111/bph.15959. Epub 2022 Oct 20. Br J Pharmacol. 2022. PMID: 36165125 Free PMC article. Review.
-
Magnitude of Drug-Drug Interactions in Special Populations.Pharmaceutics. 2022 Apr 4;14(4):789. doi: 10.3390/pharmaceutics14040789. Pharmaceutics. 2022. PMID: 35456623 Free PMC article. Review.
-
Impact of Drug Exposure on Resistance Selection Following Artemether-Lumefantrine Treatment for Malaria in Children With and Without HIV in Uganda.Clin Pharmacol Ther. 2023 Mar;113(3):660-669. doi: 10.1002/cpt.2768. Epub 2022 Nov 14. Clin Pharmacol Ther. 2023. PMID: 36260349 Free PMC article.
-
Malaria PK/PD and the Role Pharmacometrics Can Play in the Global Health Arena: Malaria Treatment Regimens for Vulnerable Populations.Clin Pharmacol Ther. 2021 Oct;110(4):926-940. doi: 10.1002/cpt.2238. Epub 2021 May 2. Clin Pharmacol Ther. 2021. PMID: 33763871 Free PMC article. Review.
References
-
- World Health Organization. World Malaria Report 2018. Geneva, Switzerland2018.
-
- World Health Organization. Number of people (all ages) living with HIV Estimates by WHO region. 2017; http://apps.who.int/gho/data/view.main.22100WHO?(2015). Accessed 10/15/2018.
-
- UNAIDS. UNAIDS DATA. Geneva Switzerland: Joint United Nations Programme on HIV/AIDS; 2018; 2018.
-
- Walker PG, ter Kuile FO, Garske T, et al. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health. 2014;2(8):e460–467. - PubMed

