Antenatal Intracellular Concentrations of Tenofovir Diphosphate and Emtricitabine Triphosphate and Associations Between Tenofovir Diphosphate and Severe Adverse Pregnancy Outcomes: IMPAACT-PROMISE (1077BF) Trial
- PMID: 31929405
- PMCID: PMC7409985
- DOI: 10.1097/QAI.0000000000002247
Antenatal Intracellular Concentrations of Tenofovir Diphosphate and Emtricitabine Triphosphate and Associations Between Tenofovir Diphosphate and Severe Adverse Pregnancy Outcomes: IMPAACT-PROMISE (1077BF) Trial
Abstract
Background: In the Promoting Maternal and Infant Survival Everywhere (PROMISE) trial, tenofovir disoproxil fumarate (TDF) use was associated with moderate or severe adverse pregnancy/neonatal outcomes. This study characterized tenofovir diphosphate (TFV-DP) and emtricitabine triphosphate (FTC-TP) concentrations in dried blood spots (DBS) and assessed association between severe adverse pregnancy/neonatal outcomes and TFV-DP concentration.
Methods: Retrospective case-control study of PROMISE trial arm-C women randomized to receive TDF, FTC, and ritonavir-boosted lopinavir (LPV/r), who took at least 1 dose of TDF + FTC and had week-4 postrandomization DBS drawn before delivery. Cases, defined as severe adverse pregnancy/neonatal outcomes (very preterm delivery before 34 weeks of gestation, stillbirth ≥20 weeks of gestation, or infant death before 14 days-of-age), were matched to controls (1:2 ratio) by site and gestational age at entry. Week 4 and week 8 DBS samples were assayed for TFV-DP and FTC-TP by liquid chromatography and tandem mass spectrometry. Associations were tested using Wilcoxon rank test and conditional logistic regression.
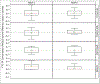
Results: Of 447 PROMISE arm-C women, 33 met case definitions, and overall, 22 cases and 44 controls were analyzed. Median (interquartile range) concentrations of TFV-DP at weeks 4 and 8 were 706 (375-1023) fmol/punch and 806 (414-1265) fmol/punch, respectively. Odds ratio (95% confidence interval) for severe adverse pregnancy/neonatal outcome with natural log of TFV-DP concentrations as the predictor were 1.27 (0.74 to 2.18) and 1.74 (0.66 to 4.60) at weeks 4 and 8, respectively. Median (interquartile range) concentrations of FTC-TP at weeks 4 and 8 were 0.27 (0.05-0.36) pmol/punch and 0.29 (0.05-0.40) pmol/punch, respectively.
Conclusions: TFV-DP concentrations in DBS appeared not to be associated with severe adverse pregnancy/neonatal outcomes, although sample size was limited.
Conflict of interest statement
Conflicts of Interest: The authors do not have financial, consultant, institutional or other relationships that might lead to a bias or conflict of interest. This work was presented in part at the 10th International Workshop on HIV Pediatrics (abstract #83), 20–21 July 2018, Amsterdam, The Netherlands.
Figures


References
-
- WHO. The Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; 2016. http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed December 14, 2018.
-
- WHO. Guidelines for the prevention, care and treatment of persons with chronic hepatitis b infection. https://www.who.int/hepatitis/publications/hepatitis-b-guidelines/en/. Published 2015. Accessed December 14, 2018. - PubMed
-
- UNAIDS. UNAIDS data 2018. http://www.unaids.org/en/resources/documents/2018/unaids-data-2018. Accessed December 15, 2018.
-
- AIDS Vaccine Advocacy Coalition (AVAC). Ongoing and Planned PrEP Open Label, Demonstration and Implementation Projects, August 2018. https://www.avac.org/sites/default/files/resource-files/ongoing_planned_.... Published 2018. Accessed December 15, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

