A non-canonical role for p27Kip1 in restricting proliferation of corneal endothelial cells during development
- PMID: 31929545
- PMCID: PMC6957298
- DOI: 10.1371/journal.pone.0226725
A non-canonical role for p27Kip1 in restricting proliferation of corneal endothelial cells during development
Abstract
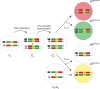
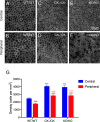
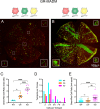
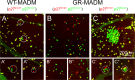
The cell cycle regulator p27Kip1 is a critical factor controlling cell number in many lineages. While its anti-proliferative effects are well-established, the extent to which this is a result of its function as a cyclin-dependent kinase (CDK) inhibitor or through other known molecular interactions is not clear. To genetically dissect its role in the developing corneal endothelium, we examined mice harboring two loss-of-function alleles, a null allele (p27-) that abrogates all protein function and a knockin allele (p27CK-) that targets only its interaction with cyclins and CDKs. Whole-animal mutants, in which all cells are either homozygous knockout or knockin, exhibit identical proliferative increases (~0.6-fold) compared with wild-type tissues. On the other hand, use of mosaic analysis with double markers (MADM) to produce infrequently-occurring clones of wild-type and mutant cells within the same tissue environment uncovers a roughly three- and six-fold expansion of individual p27CK-/CK- and p27-/- cells, respectively. Mosaicism also reveals distinct migration phenotypes, with p27-/- cells being highly restricted to their site of production and p27CK-/CK- cells more widely scattered within the endothelium. Using a density-based clustering algorithm to quantify dispersal of MADM-generated clones, a four-fold difference in aggregation is seen between the two types of mutant cells. Overall, our analysis reveals that, in developing mouse corneal endothelium, p27 regulates cell number by acting cell autonomously, both through its interactions with cyclins and CDKs and through a cyclin-CDK-independent mechanism(s). Combined with its parallel influence on cell motility, it constitutes a potent multi-functional effector mechanism with major impact on tissue organization.
Conflict of interest statement
One of us (JB), as an employee of BioVoxxel, Ludwigshafen, Germany, is engaged as a paid consultant and a developer of software that is distributed to the research community free of charge via ImageJ. This commercial affiliation does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

