Autophagy drives fibroblast senescence through MTORC2 regulation
- PMID: 31931659
- PMCID: PMC7595590
- DOI: 10.1080/15548627.2020.1713640
Autophagy drives fibroblast senescence through MTORC2 regulation
Abstract
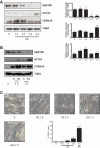
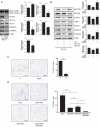
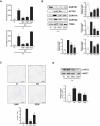
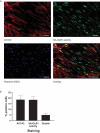
Sustained macroautophagy/autophagy favors the differentiation of fibroblasts into myofibroblasts. Cellular senescence, another means of responding to long-term cellular stress, has also been linked to myofibroblast differentiation and fibrosis. Here, we evaluate the relationship between senescence and myofibroblast differentiation in the context of sustained autophagy. We analyzed markers of cell cycle arrest/senescence in fibroblasts in vitro, where autophagy was triggered by serum starvation (SS). Autophagic fibroblasts expressed the senescence biomarkers CDKN1A/p21 and CDKN2A/p16 and exhibited increased senescence-associated GLB1/beta-galactosidase activity. Inhibition of autophagy in serum-starved fibroblasts with 3-methyladenine, LY294002, or ATG7 (autophagy related 7) silencing prevented the expression of senescence-associated markers. Similarly, suppressing MTORC2 activation using rapamycin or by silencing RICTOR also prevented senescence hallmarks. Immunofluorescence microscopy showed that senescence and myofibroblast differentiation were induced in different cells, suggesting mutually exclusive activation of senescence and myofibroblast differentiation. Reactive oxygen species (ROS) are known inducers of senescence and exposing fibroblasts to ROS scavengers decreased ROS production during SS, inhibited autophagy, and significantly reduced the expression of senescence and myofibroblast differentiation markers. ROS scavengers also curbed the AKT1 phosphorylation at Ser473, an MTORC2 target, establishing the importance of ROS in fueling MTORC2 activation. Inhibition of senescence by shRNA to TP53/p53 and shRNA CDKN2A/p16 increased myofibroblast differentiation, suggesting a negative feedback loop of senescence on autophagy-induced myofibroblast differentiation. Collectively, our results identify ROS as central inducers of MTORC2 activation during chronic autophagy, which in turn fuels senescence activation and myofibroblast differentiation in distinct cellular subpopulations. Abbreviations: 3-MA: 3-methyladenine; ACTA2: actin, alpha 2, smooth muscle, aorta; AKT1: AKT serine/threonine kinase 1; p-AKT1: AKT1 Ser473 phosphorylation; t-AKT1: total AKT serine/threonine kinase 1; ATG4A: autophagy related 4A cysteine peptidase; ATG7: autophagy gene 7; C12FDG: 5-dodecanoylaminofluorescein Di-β-D-Galactopyranoside; CDKN1A: cyclin dependent kinase inhibitor 1A; CDKN2A: cyclin dependent kinase inhibitor 2A; Ctl: control; DAPI: 4',6-diamidino-2-phenylindole, dilactate; ECM: extracellular matrix; GSH: L-glutathione reduced; H2O2: hydrogen peroxide; HLF: adult human lung fibroblasts; Ho: Hoechst 33342 (2'-[4-ethoxyphenyl]-5-[4-methyl-1-piperazinyl]-2.5'-bi-1H-benzimidazole); HSC: hepatic stellate cells; LY: LY294002; MAP1LC3B/LC3B: microtubule-associated protein 1 light chain 3 beta; MTORC1/2: mechanistic target of rapamycin kinase complex 1/2; N: normal growth medium; NAC: N-acetyl-L-cysteine; PBS: phosphate-buffered saline; PDGFA: platelet derived growth factor subunit A; PRKCA/PKCα: protein kinase C alpha; PtdIns3K: class III phosphatidylinositol 3-kinase; PTEN: phosphatase and tensin homolog; R: rapamycin; RICTOR: RPTOR independent companion of MTOR complex 2; ROS: reactive oxygen species; RPTOR: regulatory associated protein of MTOR complex 1; SA-GLB1/β-gal: senescence-associated galactosidase beta 1; SGK1: serum/glucocorticoid regulated kinase 1; shRNA: short hairpin RNA; siCtl: control siRNA; siRNA: small interfering RNA; SQSTM1: sequestosome 1; SS: serum-free (serum starvation) medium; TP53: tumor protein p53; TUBA: tubulin alpha; V: vehicle.
Keywords: Autophagy; MTORC2; myofibroblast; rapamycin; senescence.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



References
-
- Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. - PubMed
-
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. - PubMed
-
- Regulski MJ. Cellular senescence: what, why, and how. Wounds. 2017;29:168–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
