Acute severe paediatric asthma: study protocol for the development of a core outcome set, a Pediatric Emergency Reserarch Networks (PERN) study
- PMID: 31931862
- PMCID: PMC6956506
- DOI: 10.1186/s13063-019-3785-6
Acute severe paediatric asthma: study protocol for the development of a core outcome set, a Pediatric Emergency Reserarch Networks (PERN) study
Abstract
Background: Acute severe childhood asthma is an infrequent, but potentially life-threatening emergency condition. There is a wide range of different approaches to this condition, with very little supporting evidence, leading to significant variation in practice. To improve knowledge in this area, there must first be consensus on how to conduct clinical trials, so that valid comparisons can be made between future studies. We have formed an international working group comprising paediatricians and emergency physicians from North America, Europe, Asia, the Middle East, Africa, South America, Central America, Australasia and the United Kingdom.
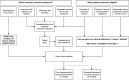
Methods/design: A 5-stage approach will be used: (1) a comprehensive list of outcomes relevant to stakeholders will be compiled through systematic reviews and qualitative interviews with patients, families, and clinicians; (2) Delphi methodology will be applied to reduce the comprehensive list to a core outcome set; (3) we will review current clinical practice guidelines, existing clinical trials, and literature on bedside assessment of asthma severity. We will then identify practice differences in tne clinical assessment of asthma severity, and determine whether further prospective work is needed to achieve agreement on inclusion criteria for clinical trials in acute paediatric asthma in the emergency department (ED) setting; (4) a retrospective chart review in Australia and New Zealand will identify the incidence of serious clinical complications such as intubation, ICU admission, and death in children hospitalized with acute severe asthma. Understanding the incidence of such outcomes will allow us to understand how common (and therefore how feasible) particular outcomes are in asthma in the ED setting; and finally (5) a meeting of the Pediatric Emergency Research Networks (PERN) asthma working group will be held, with invitation of other clinicians interested in acute asthma research, and patients/families. The group will be asked to achieve consensus on a core set of outcomes and to make recommendations for the conduct of clinical trials in acute severe asthma. If this is not possible, the group will agree on a series of prioritized steps to achieve this aim.
Discussion: The development of an international consensus on core outcomes is an important first step towards the development of consensus guidelines and standardised protocols for randomized controlled trials (RCTs) in this population. This will enable us to better interpret and compare future studies, reduce risks of study heterogeneity and outcome reporting bias, and improve the evidence base for the management of this important condition.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Acworth J, Babl F, Borland M, et al. Patterns of presentation to the Australian and New Zealand Paediatric Emergency Research Network. Emerg Med Australas. 2009;21:59–66. - PubMed
-
- Weiss AJ, Wier LM, Stocks C, Blanchard J. Overview of Emergency Department visits in the United States, 2011: statistical brief #174. Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville: Agency for Healthcare Research and Quality (US); 2006. - PubMed