Development and validation of a nomogram based on CT images and 3D texture analysis for preoperative prediction of the malignant potential in gastrointestinal stromal tumors
- PMID: 31931874
- PMCID: PMC6958787
- DOI: 10.1186/s40644-019-0284-7
Development and validation of a nomogram based on CT images and 3D texture analysis for preoperative prediction of the malignant potential in gastrointestinal stromal tumors
Abstract
Background: Gastrointestinal stromal tumors (GISTs), which are the most common mesenchymal tumors of the digestive system, are treated varyingly according to the malignancy. The purpose of this study is to develop and validate a nomogram for preoperative prediction of the malignant potential in patients with GIST.
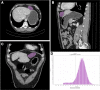
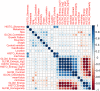
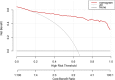
Methods: A total of 440 patients with pathologically confirmed GIST after surgery in our hospital from January 2011 to July 2019 were retrospectively analyzed. They were randomly divided into the training set (n = 308) and validation set (n = 132). CT signs and texture features of each patient were analyzed and predictive model were developed using the least absolute shrinkage and selection operator (lasso) regression. Then a nomogram based on selected parameters was developed. The predictive effectiveness of nomogram was evaluated by the area under receiver operating characteristic (ROC) curve (AUC). Concordance index (C-index) and calibration plots were formulated to evaluate the reliability and accuracy of the nomogram by bootstrapping based on internal (training set) and external (validation set) validity. The clinical application value of the nomogram was determined through the decision curve analysis (DCA).
Results: Totally 156 GIST patients with low-malignant (very low and low risk) and 284 ones with high-malignant potential (intermediate and high risk) are enrolled in this study. The prediction nomogram consisting of size, cystoid variation and meanValue had an excellent discrimination both in training and validation sets (AUCs (95% confidence interval(CI)): 0.935 (0.908, 0.961), 0.933 (0.892, 0.974); C-indices (95% CI): 0.941 (0.912, 0.956), 0.935 (0.901, 0.982); sensitivity: 81.4, 90.6%; specificity: 75.0, 75.7%; accuracy: 88.0, 88.6%, respectively). The calibration curves indicated a good consistency between the actual observation and nomogram prediction for differentiating GIST malignancy. Decision curve analysis demonstrated that the nomogram was clinically useful.
Conclusion: This study presents a prediction nomogram that incorporates the CT signs and texture parameter, which can be conveniently used to facilitate the preoperative individualized prediction of malignancy in GIST patients.
Keywords: Computed tomography; Gastrointestinal stromal tumors; Grade; Nomogram; Texture analysis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

