Quantification of tumor burden in multiple myeloma by atlas-based semi-automatic segmentation of WB-DWI
- PMID: 31931880
- PMCID: PMC6958755
- DOI: 10.1186/s40644-020-0286-5
Quantification of tumor burden in multiple myeloma by atlas-based semi-automatic segmentation of WB-DWI
Abstract
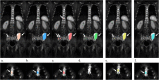
Background: Whole-body diffusion weighted imaging (WB-DWI) has proven value to detect multiple myeloma (MM) lesions. However, the large volume of imaging data and the presence of numerous lesions makes the reading process challenging. The aim of the current study was to develop a semi-automatic lesion segmentation algorithm for WB-DWI images in MM patients and to evaluate this smart-algorithm (SA) performance by comparing it to the manual segmentations performed by radiologists.
Methods: An atlas-based segmentation was developed to remove the high-signal intensity normal tissues on WB-DWI and to restrict the lesion area to the skeleton. Then, an outlier threshold-based segmentation was applied to WB-DWI images, and the segmented area's signal intensity was compared to the average signal intensity of a low-fat muscle on T1-weighted images. This method was validated in 22 whole-body DWI images of patients diagnosed with MM. Dice similarity coefficient (DSC), sensitivity and positive predictive value (PPV) were computed to evaluate the SA performance against the gold standard (GS) and to compare with the radiologists. A non-parametric Wilcoxon test was also performed. Apparent diffusion coefficient (ADC) histogram metrics and lesion volume were extracted for the GS segmentation and for the correctly identified lesions by SA and their correlation was assessed.
Results: The mean inter-radiologists DSC was 0.323 ± 0.268. The SA vs GS achieved a DSC of 0.274 ± 0.227, sensitivity of 0.764 ± 0.276 and PPV 0.217 ± 0.207. Its distribution was not significantly different from the mean DSC of inter-radiologist segmentation (p = 0.108, Wilcoxon test). ADC and lesion volume intraclass correlation coefficient (ICC) of the GS and of the correctly identified lesions by the SA was 0.996 for the median and 0.894 for the lesion volume (p < 0.001). The duration of the lesion volume segmentation by the SA was, on average, 10.22 ± 0.86 min, per patient.
Conclusions: The SA provides equally reproducible segmentation results when compared to the manual segmentation of radiologists. Thus, the proposed method offers robust and efficient segmentation of MM lesions on WB-DWI. This method may aid accurate assessment of tumor burden and therefore provide insights to treatment response assessment.
Keywords: Atlas-based segmentation; Diffusion weighted imaging; Multiple myeloma; Semi-automatic segmentation; Total lesion burden.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Messiou C, Hillengass J, Delorme S, Lecouvet FE, Moulopoulos LA, Collins DJ, et al. Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: myeloma response assessment and diagnosis system (MY-RADS) Radiology. 2019;291:5–13. doi: 10.1148/radiol.2019181949. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

