Inflammation-induced IgE promotes epithelial hyperplasia and tumour growth
- PMID: 31931959
- PMCID: PMC6959995
- DOI: 10.7554/eLife.51862
Inflammation-induced IgE promotes epithelial hyperplasia and tumour growth
Abstract
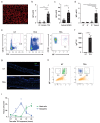
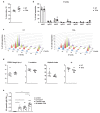
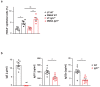
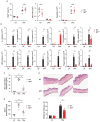
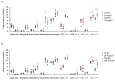
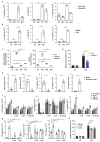
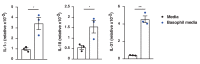
IgE is the least abundant circulating antibody class but is constitutively present in healthy tissues bound to resident cells via its high-affinity receptor, FcεRI. The physiological role of endogenous IgE antibodies is unclear but it has been suggested that they provide host protection against a variety of noxious environmental substances and parasitic infections at epithelial barrier surfaces. Here we show, in mice, that skin inflammation enhances levels of IgE antibodies that have natural specificities and a repertoire, VDJ rearrangements and CDRH3 characteristics similar to those of IgE antibodies in healthy tissue. IgE-bearing basophils are recruited to inflamed skin via CXCL12 and thymic stromal lymphopoietin (TSLP)/IL-3-dependent upregulation of CXCR4. In the inflamed skin, IgE/FcεRI-signalling in basophils promotes epithelial cell growth and differentiation, partly through histamine engagement of H1R and H4R. Furthermore, this IgE response strongly drives tumour outgrowth of epithelial cells harbouring oncogenic mutation. These findings indicate that natural IgE antibodies support skin barrier defences, but that during chronic tissue inflammation this role may be subverted to promote tumour growth.
Keywords: IgE; basophils; cancer; cancer biology; epithelial cells; immunology; inflammation; mouse; skin.
© 2020, Hayes et al.
Conflict of interest statement
MH, SW, GC, RS, WJ, DK, DV, DD, JS No competing interests declared
Figures






References
-
- Bergot AS, Ford N, Leggatt GR, Wells JW, Frazer IH, Grimbaldeston MA. HPV16-E7 expression in squamous epithelium creates a local immune suppressive environment via CCL2- and CCL5- mediated recruitment of mast cells. PLOS Pathogens. 2014;10:e1004466. doi: 10.1371/journal.ppat.1004466. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

