Phosphate Transporter PstSCAB of Campylobacter jejuni Is a Critical Determinant of Lactate-Dependent Growth and Colonization in Chickens
- PMID: 31932316
- PMCID: PMC7167465
- DOI: 10.1128/JB.00716-19
Phosphate Transporter PstSCAB of Campylobacter jejuni Is a Critical Determinant of Lactate-Dependent Growth and Colonization in Chickens
Abstract
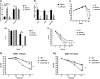
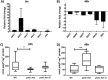
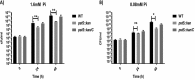
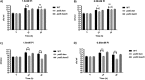
Campylobacter jejuni causes acute gastroenteritis worldwide and is transmitted primarily through poultry, in which it is often a commensal member of the intestinal microbiota. Previous transcriptome sequencing (RNA-Seq) experiment showed that transcripts from an operon encoding a high-affinity phosphate transporter (PstSCAB) of C. jejuni were among the most abundant when the bacterium was grown in chickens. Elevated levels of the pstSCAB mRNA were also identified in an RNA-Seq experiment from human infection studies. In this study, we explore the role of PstSCAB in the biology and colonization potential of C. jejuni Our results demonstrate that cells lacking PstSCAB survive poorly in stationary phase, in nutrient-limiting media, and under osmotic conditions reflective of those in the chicken. Polyphosphate levels in the mutant cells were elevated at stationary phase, consistent with alterations in expression of polyphosphate metabolism genes. The mutant strain was highly attenuated for colonization of newly hatched chicks, with levels of bacteria at several orders of magnitude below wild-type levels. Mutant and wild type grew similarly in complex media, but the pstS::kan mutant exhibited a significant growth defect in minimal medium supplemented with l-lactate, postulated as a carbon source in vivo Poor growth in lactate correlated with diminished expression of acetogenesis pathway genes previously demonstrated as important for colonizing chickens. The phosphate transport system is thus essential for diverse aspects of C. jejuni physiology and in vivo fitness and survival.IMPORTANCECampylobacter jejuni causes millions of human gastrointestinal infections annually, with poultry a major source of infection. Due to the emergence of multidrug resistance in C. jejuni, there is need to identify alternative ways to control this pathogen. Genes encoding the high-affinity phosphate transporter PstSCAB are highly expressed by C. jejuni in chickens and humans. In this study, we address the role of PstSCAB on chicken colonization and other C. jejuni phenotypes. PstSCAB is required for colonization in chicken, metabolism and survival under different stress responses, and during growth on lactate, a potential growth substrate in chickens. Our study highlights that PstSCAB may be an effective target to develop mechanisms for controlling bacterial burden in both chicken and human.
Keywords: Campylobacter jejuni; host-pathogen interactions; phosphate metabolism.
Copyright © 2020 American Society for Microbiology.
Figures



Similar articles
-
Influence of Campylobacter jejuni fliA, rpoN and flgK genes on colonization of the chicken gut.Int J Food Microbiol. 2007 Sep 15;118(2):194-200. doi: 10.1016/j.ijfoodmicro.2007.07.038. Epub 2007 Aug 1. Int J Food Microbiol. 2007. PMID: 17761334
-
Campylobacter jejuni influences the expression of nutrient transporter genes in the intestine of chickens.Vet Microbiol. 2014 Aug 6;172(1-2):195-201. doi: 10.1016/j.vetmic.2014.04.001. Epub 2014 Apr 13. Vet Microbiol. 2014. PMID: 24834798
-
Re-thinking the chicken-Campylobacter jejuni interaction: a review.Avian Pathol. 2018 Aug;47(4):352-363. doi: 10.1080/03079457.2018.1475724. Epub 2018 Jun 11. Avian Pathol. 2018. PMID: 29764197 Review.
-
Evaluation of passive immunotherapeutic efficacy of hyperimmunized egg yolk powder against intestinal colonization of Campylobacter jejuni in chickens.Poult Sci. 2014 Nov;93(11):2779-87. doi: 10.3382/ps.2014-04234. Epub 2014 Sep 11. Poult Sci. 2014. PMID: 25214556
-
Colonization factors of Campylobacter jejuni in the chicken gut.Vet Res. 2011 Jun 29;42(1):82. doi: 10.1186/1297-9716-42-82. Vet Res. 2011. PMID: 21714866 Free PMC article. Review.
Cited by
-
Therapeutic effects of oral benzoic acid application during acute murine campylobacteriosis.Eur J Microbiol Immunol (Bp). 2024 May 27;14(3):243-260. doi: 10.1556/1886.2024.00059. Print 2024 Sep 11. Eur J Microbiol Immunol (Bp). 2024. PMID: 38801662 Free PMC article.
-
Trivalent outer membrane vesicles-based combination vaccine candidate induces protective immunity against Campylobacter and invasive non-typhoidal Salmonella in adult mice.Med Microbiol Immunol. 2024 Oct 15;213(1):21. doi: 10.1007/s00430-024-00805-z. Med Microbiol Immunol. 2024. PMID: 39407046
-
Fitness factor genes conserved within the multi-species core genome of Gram-negative Enterobacterales species contribute to bacteremia pathogenesis.PLoS Pathog. 2024 Aug 23;20(8):e1012495. doi: 10.1371/journal.ppat.1012495. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39178317 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

