HSD3B1 genotype identifies glucocorticoid responsiveness in severe asthma
- PMID: 31932420
- PMCID: PMC6995013
- DOI: 10.1073/pnas.1918819117
HSD3B1 genotype identifies glucocorticoid responsiveness in severe asthma
Abstract
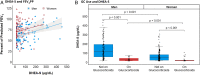
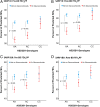
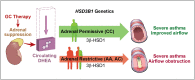
Asthma resistance to glucocorticoid treatment is a major health problem with unclear etiology. Glucocorticoids inhibit adrenal androgen production. However, androgens have potential benefits in asthma. HSD3B1 encodes for 3β-hydroxysteroid dehydrogenase-1 (3β-HSD1), which catalyzes peripheral conversion from adrenal dehydroepiandrosterone (DHEA) to potent androgens and has a germline missense-encoding polymorphism. The adrenal restrictive HSD3B1(1245A) allele limits conversion, whereas the adrenal permissive HSD3B1(1245C) allele increases DHEA metabolism to potent androgens. In the Severe Asthma Research Program (SARP) III cohort, we determined the association between DHEA-sulfate and percentage predicted forced expiratory volume in 1 s (FEV1PP). HSD3B1(1245) genotypes were assessed, and association between adrenal restrictive and adrenal permissive alleles and FEV1PP in patients with (GC) and without (noGC) daily oral glucocorticoid treatment was determined (n = 318). Validation was performed in a second cohort (SARP I&II; n = 184). DHEA-sulfate is associated with FEV1PP and is suppressed with GC treatment. GC patients homozygous for the adrenal restrictive genotype have lower FEV1PP compared with noGC patients (54.3% vs. 75.1%; P < 0.001). In patients with the homozygous adrenal permissive genotype, there was no FEV1PP difference in GC vs. noGC patients (73.4% vs. 78.9%; P = 0.39). Results were independently confirmed: FEV1PP for homozygous adrenal restrictive genotype in GC vs. noGC is 49.8 vs. 63.4 (P < 0.001), and for homozygous adrenal permissive genotype, it is 66.7 vs. 67.7 (P = 0.92). The adrenal restrictive HSD3B1(1245) genotype is associated with GC resistance. This effect appears to be driven by GC suppression of 3β-HSD1 substrate. Our results suggest opportunities for prediction of GC resistance and pharmacologic intervention.
Keywords: HSD3B1; androgens; glucocorticoids; inflammation; steroids.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: Cleveland Clinic has applied for patents on HSD3B1.
Figures
References
-
- Hench P. S., Slocumb C. H., Polley H. F., Kendal E. C., Effect of cortisone and pituitary adrenocorticotropic hormone (ACTH) on rheumatic diseases. J. Am. Med. Assoc. 144, 1327–1335 (1950). - PubMed
-
- Barnes P. J., Adcock I. M., Glucocorticoid resistance in inflammatory diseases. Lancet 373, 1905–1917 (2009). - PubMed
-
- Chung K. F., et al. , International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 43, 343–373 (2014) Erratum in: Eur Respir J. 43, 1216 (2014). - PubMed
-
- Mendoza-Milla C., et al. , Dehydroepiandrosterone has strong antifibrotic effects and is decreased in idiopathic pulmonary fibrosis. Eur. Respir. J. 42, 1309–1321 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

