Therapy-Induced Senescence Drives Bone Loss
- PMID: 31932453
- PMCID: PMC7056549
- DOI: 10.1158/0008-5472.CAN-19-2348
Therapy-Induced Senescence Drives Bone Loss
Abstract
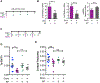
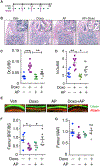
Chemotherapy is important for cancer treatment, however, toxicities limit its use. While great strides have been made to ameliorate the acute toxicities induced by chemotherapy, long-term comorbidities including bone loss remain a significant problem. Chemotherapy-driven estrogen loss is postulated to drive bone loss, but significant data suggests the existence of an estrogen-independent mechanism of bone loss. Using clinically relevant mouse models, we showed that senescence and its senescence-associated secretory phenotype (SASP) contribute to chemotherapy-induced bone loss that can be rescued by depleting senescent cells. Chemotherapy-induced SASP could be limited by targeting the p38MAPK-MK2 pathway, which resulted in preservation of bone integrity in chemotherapy-treated mice. These results transform our understanding of chemotherapy-induced bone loss by identifying senescent cells as major drivers of bone loss and the p38MAPK-MK2 axis as a putative therapeutic target that can preserve bone and improve a cancer survivor's quality of life. SIGNIFICANCE: Senescence drives chemotherapy-induced bone loss that is rescued by p38MAPK or MK2 inhibitors. These findings may lead to treatments for therapy-induced bone loss, significantly increasing quality of life for cancer survivors.
©2020 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest
JBM is the Executive Vice President of R&D of Aclaris Therapeutics, Inc. JvD and DJB are co-inventors on patent applications licensed to or filed by Unity Biotechnology, a company developing senolytic medicines, including small molecules that selectively eliminate senescent cells. JvD is a co-founder of Unity Biotechnology. The other authors have no competing interests to declare.
Figures





References
-
- Chao C, Studts JL, Abell T, Hadley T, Roetzer L, Dineen S, et al. Adjuvant chemotherapy for breast cancer: how presentation of recurrence risk influences decision-making. J Clin Oncol 2003;21:4299–305 - PubMed
-
- Ishitobi M, Komoike Y, Motomura K, Koyama H, Inaji H. Early response to neo-adjuvant chemotherapy in carcinoma of the breast predicts both successful breast-conserving surgery and decreased risk of ipsilateral breast tumor recurrence. Breast J 2010;16:9–13 - PubMed
-
- Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins L, Lower E, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 2005;23:5542–51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

