The Kidney Contains Ontogenetically Distinct Dendritic Cell and Macrophage Subtypes throughout Development That Differ in Their Inflammatory Properties
- PMID: 31932472
- PMCID: PMC7003301
- DOI: 10.1681/ASN.2019040419
The Kidney Contains Ontogenetically Distinct Dendritic Cell and Macrophage Subtypes throughout Development That Differ in Their Inflammatory Properties
Abstract
Background: Mononuclear phagocytes (MPs), including macrophages, monocytes, and dendritic cells (DCs), are phagocytic cells with important roles in immunity. The developmental origin of kidney DCs has been highly debated because of the large phenotypic overlap between macrophages and DCs in this tissue.
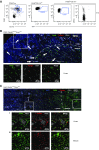
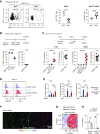
Methods: We used fate mapping, RNA sequencing, flow cytometry, confocal microscopy, and histo-cytometry to assess the origin and phenotypic and functional properties of renal DCs in healthy kidney and of DCs after cisplatin and ischemia reperfusion-induced kidney injury.
Results: Adult kidney contains at least four subsets of MPs with prominent Clec9a-expression history indicating a DC origin. We demonstrate that these populations are phenotypically, functionally, and transcriptionally distinct from each other. We also show these kidney MPs exhibit unique age-dependent developmental heterogeneity. Kidneys from newborn mice contain a prominent population of embryonic-derived MHCIInegF4/80hiCD11blow macrophages that express T cell Ig and mucin domain containing 4 (TIM-4) and MER receptor tyrosine kinase (MERTK). These macrophages are replaced within a few weeks after birth by phenotypically similar cells that express MHCII but lack TIM-4 and MERTK. MHCII+F4/80hi cells exhibit prominent Clec9a-expression history in adulthood but not early life, indicating additional age-dependent developmental heterogeneity. In AKI, MHCIInegF4/80hi cells reappear in adult kidneys as a result of MHCII downregulation by resident MHCII+F4/80hi cells, possibly in response to prostaglandin E2 (PGE2). RNA sequencing further suggests MHCII+F4/80hi cells help coordinate the recruitment of inflammatory cells during renal injury.
Conclusions: Distinct developmental programs contribute to renal DC and macrophage populations throughout life, which could have important implications for therapies targeting these cells.
Keywords: acute kidney injury; dendritic cell; hematopoiesis; immunology; kidney development; macrophages.
Copyright © 2020 by the American Society of Nephrology.
Figures







Comment in
-
Unraveling the Complexity of the Renal Mononuclear Phagocyte System by Genetic Cell Lineage Tracing.J Am Soc Nephrol. 2020 Feb;31(2):233-235. doi: 10.1681/ASN.2019121295. Epub 2020 Jan 13. J Am Soc Nephrol. 2020. PMID: 31949050 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

