A Redox-Sensitive Thiol in Wis1 Modulates the Fission Yeast Mitogen-Activated Protein Kinase Response to H2O2 and Is the Target of a Small Molecule
- PMID: 31932483
- PMCID: PMC7076255
- DOI: 10.1128/MCB.00346-19
A Redox-Sensitive Thiol in Wis1 Modulates the Fission Yeast Mitogen-Activated Protein Kinase Response to H2O2 and Is the Target of a Small Molecule
Abstract
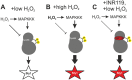
Oxidation of a highly conserved cysteine (Cys) residue located in the kinase activation loop of mitogen-activated protein kinase kinases (MAPKK) inactivates mammalian MKK6. This residue is conserved in the fission yeast Schizosaccharomyces pombe MAPKK Wis1, which belongs to the H2O2-responsive MAPK Sty1 pathway. Here, we show that H2O2 reversibly inactivates Wis1 through this residue (C458) in vitro We found that C458 is oxidized in vivo and that serine replacement of this residue significantly enhances Wis1 activation upon addition of H2O2 The allosteric MAPKK inhibitor INR119, which binds in a pocket next to the activation loop and C458, prevented the inhibition of Wis1 by H2O2in vitro and significantly increased Wis1 activation by low levels of H2O2in vivo We propose that oxidation of C458 inhibits Wis1 and that INR119 cancels out this inhibitory effect by binding close to this residue. Kinase inhibition through the oxidation of a conserved Cys residue in MKK6 (C196) is thus conserved in the S. pombe MAPKK Wis1.
Keywords: Schizosaccharomyces pombe; Sty1; cysteine oxidation; stress signaling.
Copyright © 2020 Sjölander et al.
Figures





References
-
- Samejima I, Mackie S, Warbrick E, Weisman R, Fantes PA. 1998. The fission yeast mitotic regulator win1+ encodes an MAP kinase kinase kinase that phosphorylates and activates Wis1 MAP kinase kinase in response to high osmolarity. Mol Biol Cell 9:2325–2335. doi: 10.1091/mbc.9.8.2325. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
