Phasic Dopamine Release Magnitude Tracks Individual Differences in Sensitization of Locomotor Response following a History of Nicotine Exposure
- PMID: 31932634
- PMCID: PMC6957501
- DOI: 10.1038/s41598-019-56884-z
Phasic Dopamine Release Magnitude Tracks Individual Differences in Sensitization of Locomotor Response following a History of Nicotine Exposure
Abstract
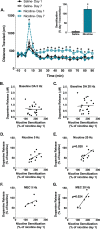
Smoking remains the primary cause of preventable death in the United States and smoking related illness costs more than $300 billion annually. Nicotine (the primary reinforcer in cigarettes) causes changes in behavior and neurochemistry that lead to increased probability of relapse. Given the role of mesolimbic dopamine projections in motivation, substance use disorder, and drug relapse, we examined the effect of repeated nicotine on rapid dopamine signals in the nucleus accumbens (NAc) of rats. Adult, male Sprague-Dawley rats were exposed to nicotine (0.2 or 0.4 mg/kg, subcutaneous) once daily for 7 days. On day 8, dopamine release and uptake dynamics, and their modulation by nicotinic receptor agonists and antagonists, were assessed using fast scan cyclic voltammetry in the NAc core. Nicotine exposure decreased electrically-stimulated dopamine release across a range of stimulation frequencies and decreased α6β2-containing nicotinic receptor control over dopamine release. Additionally, nicotine locomotor sensitization correlated with accumbal dopamine modulation by nicotine and mecamylamine. Taken together, our study suggests that repeated exposure to nicotine blunts dopamine release in the NAc core through changes in α6β2 modulation of dopamine release and individual differences in the sensitivity to this outcome may predict variation in behavioral models of vulnerability to substance use disorder.
Conflict of interest statement
The authors declare no competing interests.
Figures

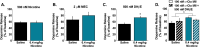

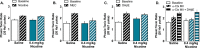
Similar articles
-
α6β2 subunit containing nicotinic acetylcholine receptors exert opposing actions on rapid dopamine signaling in the nucleus accumbens of rats with high-versus low-response to novelty.Neuropharmacology. 2017 Nov;126:281-291. doi: 10.1016/j.neuropharm.2017.06.028. Epub 2017 Jun 27. Neuropharmacology. 2017. PMID: 28666811 Free PMC article.
-
Long-term nicotine treatment down-regulates α6β2* nicotinic receptor expression and function in nucleus accumbens.J Neurochem. 2013 Dec;127(6):762-71. doi: 10.1111/jnc.12442. Epub 2013 Oct 13. J Neurochem. 2013. PMID: 23992036 Free PMC article.
-
Long-term effects of adolescent versus adult nicotine self-administration on cholinergic modulation of dopamine in the nucleus accumbens core.Drug Alcohol Depend. 2025 Mar 1;268:112555. doi: 10.1016/j.drugalcdep.2025.112555. Epub 2025 Jan 15. Drug Alcohol Depend. 2025. PMID: 39881470
-
Sensitization of the mesoaccumbens dopamine response to nicotine.Pharmacol Biochem Behav. 1998 Apr;59(4):1021-30. doi: 10.1016/s0091-3057(97)00537-6. Pharmacol Biochem Behav. 1998. PMID: 9586863 Review.
-
Plasticity of addiction: a mesolimbic dopamine short-circuit?Am J Addict. 2009 Jul-Aug;18(4):259-71. doi: 10.1080/10550490902925946. Am J Addict. 2009. PMID: 19444729 Free PMC article. Review.
Cited by
-
A mechanistic model of ADHD as resulting from dopamine phasic/tonic imbalance during reinforcement learning.Front Comput Neurosci. 2022 Jul 18;16:849323. doi: 10.3389/fncom.2022.849323. eCollection 2022. Front Comput Neurosci. 2022. PMID: 35923915 Free PMC article.
-
Implications of Kynurenine Pathway Metabolism for the Immune System, Hypothalamic-Pituitary-Adrenal Axis, and Neurotransmission in Alcohol Use Disorder.Int J Mol Sci. 2024 Apr 29;25(9):4845. doi: 10.3390/ijms25094845. Int J Mol Sci. 2024. PMID: 38732064 Free PMC article. Review.
-
Subregion specific neuroadaptations in the female rat striatum during acute and protracted withdrawal from nicotine.J Neural Transm (Vienna). 2024 Jan;131(1):83-94. doi: 10.1007/s00702-023-02678-7. Epub 2023 Jul 27. J Neural Transm (Vienna). 2024. PMID: 37500938 Free PMC article.
-
α7 nicotinic acetylcholine receptor modulation of accumbal dopamine release covaries with novelty seeking.Eur J Neurosci. 2022 Mar;55(5):1162-1173. doi: 10.1111/ejn.15620. Epub 2022 Feb 22. Eur J Neurosci. 2022. PMID: 35141983 Free PMC article.
-
Pharmacokinetic differences in nicotine and nicotine salts mediate reinforcement-related behavior: an animal model study.Front Neurosci. 2023 Nov 16;17:1288102. doi: 10.3389/fnins.2023.1288102. eCollection 2023. Front Neurosci. 2023. PMID: 38033549 Free PMC article.
References
-
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health (2014).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

