Annexin-V stabilizes membrane defects by inducing lipid phase transition
- PMID: 31932647
- PMCID: PMC6957514
- DOI: 10.1038/s41467-019-14045-w
Annexin-V stabilizes membrane defects by inducing lipid phase transition
Abstract
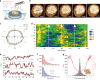
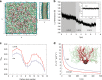
Annexins are abundant cytoplasmic proteins, which bind to membranes that expose negatively charged phospholipids in a Ca2+-dependent manner. During cell injuries, the entry of extracellular Ca2+ activates the annexin membrane-binding ability, subsequently initiating membrane repair processes. However, the mechanistic action of annexins in membrane repair remains largely unknown. Here, we use high-speed atomic force microscopy (HS-AFM), fluorescence recovery after photobleaching (FRAP), confocal laser scanning microscopy (CLSM) and molecular dynamics simulations (MDSs) to analyze how annexin-V (A5) binds to phosphatidylserine (PS)-rich membranes leading to high Ca2+-concentrations at membrane, and then to changes in the dynamics and organization of lipids, eventually to a membrane phase transition. A5 self-assembly into lattices further stabilizes and likely structures the membrane into a gel phase. Our findings are compatible with the patch resealing through vesicle fusion mechanism in membrane repair and indicate that A5 retains negatively charged lipids in the inner leaflet in an injured cell.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

