ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer
- PMID: 31932695
- PMCID: PMC7341683
- DOI: 10.1038/s41588-019-0554-0
ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer
Abstract
Mutations in ARID1A, a subunit of the SWI/SNF chromatin remodeling complex, are the most common alterations of the SWI/SNF complex in estrogen-receptor-positive (ER+) breast cancer. We identify that ARID1A inactivating mutations are present at a high frequency in advanced endocrine-resistant ER+ breast cancer. An epigenome CRISPR-CAS9 knockout (KO) screen identifies ARID1A as the top candidate whose loss determines resistance to the ER degrader fulvestrant. ARID1A inactivation in cells and in patients leads to resistance to ER degraders by facilitating a switch from ER-dependent luminal cells to ER-independent basal-like cells. Cellular plasticity is mediated by loss of ARID1A-dependent SWI/SNF complex targeting to genomic sites of the luminal lineage-determining transcription factors including ER, forkhead box protein A1 (FOXA1) and GATA-binding factor 3 (GATA3). ARID1A also regulates genome-wide ER-FOXA1 chromatin interactions and ER-dependent transcription. Altogether, we uncover a critical role for ARID1A in maintaining luminal cell identity and endocrine therapeutic response in ER+ breast cancer.
Conflict of interest statement
Competing interests
M.Scaltriti has received research funds from Puma Biotechnology, Daiichi Sankyo, Immunomedics, TargImmune Therapeutics and Menarini Ricerche, is a cofounder of Medendi Medical Travel and is on the advisory board of Menarini Ricerche. C.K. is a scientific founder, fiduciary Board of Directors member, Scientific Advisory Board member, shareholder and consultant for Foghorn Therapeutics. R.L.L. is on the supervisory board of QIAGEN and is a scientific advisor to Loxo Oncology, Imago, C4 Therapeutics and Isoplexis, each including an equity interest. He receives research support from and consulted for Celgene and Roche, has received research support from Prelude Therapeutics and has consulted for Incyte, Novartis, MorphoSys and Janssen. He has received honoraria from Eli Lilly and Amgen for invited lectures and from Gilead Sciences for grant reviews. J.B. is an employee and shareholder of AstraZeneca, Board of Directors member of Foghorn Therapeutics and is a past board member of Varian Medical Systems, Bristol-Myers Squibb, Grail, Aura Biosciences and Infinity Pharmaceuticals. He has performed consulting and/or advisory work for Grail, PMV Pharma, ApoGen Biotechnologies, Juno, Eli Lilly, Seragon Pharmaceuticals, Novartis and Northern Biologics. He has stock or other ownership interests in PMV Pharma, Grail, Juno, Varian Medical Systems, Foghorn Therapeutics, Aura Biosciences, Infinity Pharmaceuticals and ApoGen Biotechnologies, as well as Tango Therapeutics and Venthera, of which he is a cofounder. He has previously received honoraria or travel expenses from Roche, Novartis and Eli Lilly. P.R. has received consultation fees from Novartis and institutional research funds from Grail and Illumina. J.S.R. is a consultant of Goldman Sachs and Repare Therapeutics, a member of the Scientific Advisory Board of VolitionRx and Paige (Artificial Intelligence) and an ad hoc member of the Scientific Advisory Board of Ventana Medical Systems, Roche, Genentech, Novartis and InviCRO, outside of the scope of the submitted work. E.T. has received honoraria from AstraZeneca for invited lectures. No potential conflicts of interests were disclosed by the other authors.
Figures

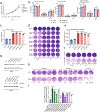



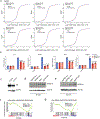
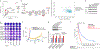





References
-
- Perou CM et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

