Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer's disease
- PMID: 31932797
- PMCID: PMC6980793
- DOI: 10.1038/s41591-019-0695-9
Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer's disease
Erratum in
-
Author Correction: Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer's disease.Nat Med. 2020 Jun;26(6):981. doi: 10.1038/s41591-020-0922-4. Nat Med. 2020. PMID: 32409732
Abstract
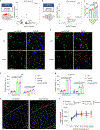
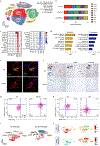
Glia have been implicated in Alzheimer's disease (AD) pathogenesis. Variants of the microglia receptor triggering receptor expressed on myeloid cells 2 (TREM2) increase AD risk, and activation of disease-associated microglia (DAM) is dependent on TREM2 in mouse models of AD. We surveyed gene-expression changes associated with AD pathology and TREM2 in 5XFAD mice and in human AD by single-nucleus RNA sequencing. We confirmed the presence of Trem2-dependent DAM and identified a previously undiscovered Serpina3n+C4b+ reactive oligodendrocyte population in mice. Interestingly, remarkably different glial phenotypes were evident in human AD. Microglia signature was reminiscent of IRF8-driven reactive microglia in peripheral-nerve injury. Oligodendrocyte signatures suggested impaired axonal myelination and metabolic adaptation to neuronal degeneration. Astrocyte profiles indicated weakened metabolic coordination with neurons. Notably, the reactive phenotype of microglia was less evident in TREM2-R47H and TREM2-R62H carriers than in non-carriers, demonstrating a TREM2 requirement in both mouse and human AD, despite the marked species-specific differences.
Conflict of interest statement
Competing Interests Statement
M. Colonna receives research support from Pfizer, Amgen, Alector and Ono.
Figures














References
Additional references for online methods and supplementary material
-
- Geiss GK et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol 26, 317–325 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

