Programmed 'disarming' of the neutrophil proteome reduces the magnitude of inflammation
- PMID: 31932813
- PMCID: PMC7223223
- DOI: 10.1038/s41590-019-0571-2
Programmed 'disarming' of the neutrophil proteome reduces the magnitude of inflammation
Abstract
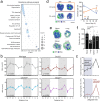
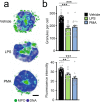
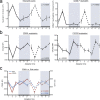
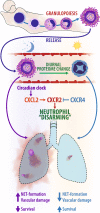
The antimicrobial functions of neutrophils are facilitated by a defensive armamentarium of proteins stored in granules, and by the formation of neutrophil extracellular traps (NETs). However, the toxic nature of these structures poses a threat to highly vascularized tissues, such as the lungs. Here, we identified a cell-intrinsic program that modified the neutrophil proteome in the circulation and caused the progressive loss of granule content and reduction of the NET-forming capacity. This program was driven by the receptor CXCR2 and by regulators of circadian cycles. As a consequence, lungs were protected from inflammatory injury at times of day or in mouse mutants in which granule content was low. Changes in the proteome, granule content and NET formation also occurred in human neutrophils, and correlated with the incidence and severity of respiratory distress in pneumonia patients. Our findings unveil a 'disarming' strategy of neutrophils that depletes protein stores to reduce the magnitude of inflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures








Comment in
-
Of larks and owls.Nat Immunol. 2020 Feb;21(2):104-105. doi: 10.1038/s41590-019-0579-7. Nat Immunol. 2020. PMID: 31932811 Free PMC article. No abstract available.
References
-
- Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 2016;273:11–28. - PubMed
-
- Ley K, et al. Neutrophils: new insights and open questions. Sci. Immunol. 2018;3:eaat4579. - PubMed
-
- Borregaard N, Sørensen OE, Theilgaard-Mönch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. - PubMed
-
- Rørvig S, Østergaard O, Heegaard NH, Borregaard N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. J. Leukoc. Biol. 2013;94:711–721. - PubMed
-
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

