Molecular MRD status and outcome after transplantation in NPM1-mutated AML
- PMID: 31932839
- PMCID: PMC7059484
- DOI: 10.1182/blood.2019002959
Molecular MRD status and outcome after transplantation in NPM1-mutated AML
Abstract
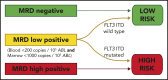
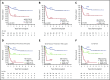
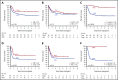
Relapse remains the most common cause of treatment failure for patients with acute myeloid leukemia (AML) who undergo allogeneic stem cell transplantation (alloSCT), and carries a grave prognosis. Multiple studies have identified the presence of measurable residual disease (MRD) assessed by flow cytometry before alloSCT as a strong predictor of relapse, but it is not clear how these findings apply to patients who test positive in molecular MRD assays, which have far greater sensitivity. We analyzed pretransplant blood and bone marrow samples by reverse-transcription polymerase chain reaction in 107 patients with NPM1-mutant AML enrolled in the UK National Cancer Research Institute AML17 study. After a median follow-up of 4.9 years, patients with negative, low (<200 copies per 105ABL in the peripheral blood and <1000 copies in the bone marrow aspirate), and high levels of MRD had an estimated 2-year overall survival (2y-OS) of 83%, 63%, and 13%, respectively (P < .0001). Focusing on patients with low-level MRD before alloSCT, those with FLT3 internal tandem duplications(ITDs) had significantly poorer outcome (hazard ratio [HR], 6.14; P = .01). Combining these variables was highly prognostic, dividing patients into 2 groups with 2y-OS of 17% and 82% (HR, 13.2; P < .0001). T-depletion was associated with significantly reduced survival both in the entire cohort (2y-OS, 56% vs 96%; HR, 3.24; P = .0005) and in MRD-positive patients (2y-OS, 34% vs 100%; HR, 3.78; P = .003), but there was no significant effect of either conditioning regimen or donor source on outcome. Registered at ISRCTN (http://www.isrctn.com/ISRCTN55675535).
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Burnett AK, Goldstone A, Hills RK, et al. . Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol. 2013;31(10):1293-1301. - PubMed
-
- Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. - PubMed
-
- Ivey A, Hills RK, Simpson MA, et al. ; UK National Cancer Research Institute AML Working Group . Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374(5):422-433. - PubMed
-
- Balsat M, Renneville A, Thomas X, et al. . Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the Acute Leukemia French Association Group. J Clin Oncol. 2017;35(2):185-193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

