Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML
- PMID: 31932844
- PMCID: PMC7068032
- DOI: 10.1182/blood.2019003988
Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML
Abstract
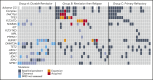
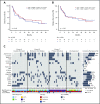
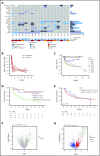
The BCL-2 inhibitor venetoclax combined with hypomethylating agents or low-dose cytarabine represents an important new therapy for older or unfit patients with acute myeloid leukemia (AML). We analyzed 81 patients receiving these venetoclax-based combinations to identify molecular correlates of durable remission, response followed by relapse (adaptive resistance), or refractory disease (primary resistance). High response rates and durable remissions were typically associated with NPM1 or IDH2 mutations, with prolonged molecular remissions prevalent for NPM1 mutations. Primary and adaptive resistance to venetoclax-based combinations was most commonly characterized by acquisition or enrichment of clones activating signaling pathways such as FLT3 or RAS or biallelically perturbing TP53. Single-cell studies highlighted the polyclonal nature of intratumoral resistance mechanisms in some cases. Among cases that were primary refractory, we identified heterogeneous and sometimes divergent interval changes in leukemic clones within a single cycle of therapy, highlighting the dynamic and rapid occurrence of therapeutic selection in AML. In functional studies, FLT3 internal tandem duplication gain or TP53 loss conferred cross-resistance to both venetoclax and cytotoxic-based therapies. Collectively, we highlight molecular determinants of outcome with clinical relevance to patients with AML receiving venetoclax-based combination therapies.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: I.J.M., D.C.S.H., and A.W.R. are employees and A.W. a former employee of the Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax. I.J.M., D.C.S.H., A.W.R., and A.H.W. received payments from the Walter and Eliza Hall Institute related to venetoclax. A.H.W. is a medical advisor and receives research funding and honoraria from Abbvie. C.D.D. has received research funding from AbbVie/Genentech, Agios, Celgene, and Daiichi Sankyo and served in a consultancy/advisory role for AbbVie, Agios, Celgene, Daiichi-Sankyo, Jazz and Notable Labs.
Figures



Comment in
-
BCL-2 inhibition and AML: can we best Darwin?Blood. 2020 Mar 12;135(11):781-782. doi: 10.1182/blood.2019004757. Blood. 2020. PMID: 32163555 No abstract available.
References
-
- Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114-1124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

