Toxicity evaluation of 6-mercaptopurine-Chitosan nanoparticles in rats
- PMID: 31933529
- PMCID: PMC6950973
- DOI: 10.1016/j.jsps.2019.11.018
Toxicity evaluation of 6-mercaptopurine-Chitosan nanoparticles in rats
Abstract
Background: The 6-mercaptopurine (6-MP) is an effective immunosuppressant and anti-cancer drug. However, the usage of 6-MP is limited due to its well-known side effects, such as myelotoxicity and hepato-renal toxicity. To curtail the potential toxic effects, we have used chitosan as a natural biodegradable and biocompatible polysaccharide to synthesize 6-Mercaptopurine-Chitosan Nanoparticles (6-MP-CNPs).
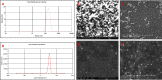
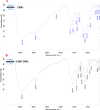
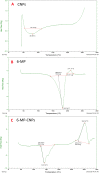
Methods: The 6-MP-CNPssize, morphology, physicochemical interactions, and thermal stability were characterized using Dynamic Light Scattering (DLS), Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), and Differential Scanning Calorimetry (DSC), respectively. The loading efficiency of the 6-MP in CNPs was estimated using LCMS/MS. Then, the 6-MP-CNPs were subjected to in vivo acute and sub-acute oral toxicity evaluations.
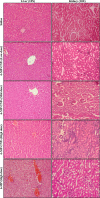
Results: The DLS and SEM analysis respectively indicated size (70.0 nm to 400.0 nm), polydispersity index (0.462), and zeta potential (54.9 mV) with improved morphology of 6-MP-CNPs. The FTIR and DSC results showed the efficient interactive and stable nature of the 6-MP-CNPs, which sustained the drug-delivery process. The loading efficiency of 6-MP-CNPs was found to be 25.23%. The chitosan improved the lethal dose (LD50 cut off) of 6-MP-CNPs (1000 mg/kg b.w) against 6-MP (500 mg/kg b.w) and also significantly (p ≤ 0.05) reduces the toxic adverse effect (28-day repeated oral dose) on hemato-biochemical and hepato-renal histological profiles.
Conclusion: The findings suggest that chitosan, as a prime drug-delivery carrier, significantly alleviates the acute and sub-acute toxic effects of 6-MP.
Keywords: 6-Mercaptopurine; Anti-cancer; Chitosan; Nanoparticle; Toxicity.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Adam de Beaumais T., Fakhoury M., Medard Y., Azougagh S., Zhang D., Yakouben K., Jacqz-Aigrain E. Determinants of mercaptopurine toxicity in pediatric acute lymphoblastic leukemia maintenance therapy. Br. J. Clin. Pharmacol. 2011;71:575–584. doi: 10.1111/j.1365-2125.2010.03867.x. - DOI - PMC - PubMed
-
- Atyabi F., Talaie F., Dinarvand R. Thiolated chitosan nanoparticles as an oral delivery system for Amikacin: in vitro and ex vivo evaluations. J. Nanosci. Nanotechnol. 2009;9:4593–4603. - PubMed
-
- Benkov K., Lu Y., Patel A., Rahhal R., Russell G., Teitelbaum J., Naspghan Committee on Inflammatory Bowel Disease Role of thiopurine metabolite testing and thiopurine methyltransferase determination in pediatric IBD. J. Pediatr. Gastroenterol. Nutr. 2013;56:333–340. doi: 10.1097/MPG.0b013e3182844705. - DOI - PubMed
-
- Berger J., Reist M., Mayer J.M., Felt O., Peppas N.A., Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004;57:19–34. - PubMed
LinkOut - more resources
Full Text Sources

